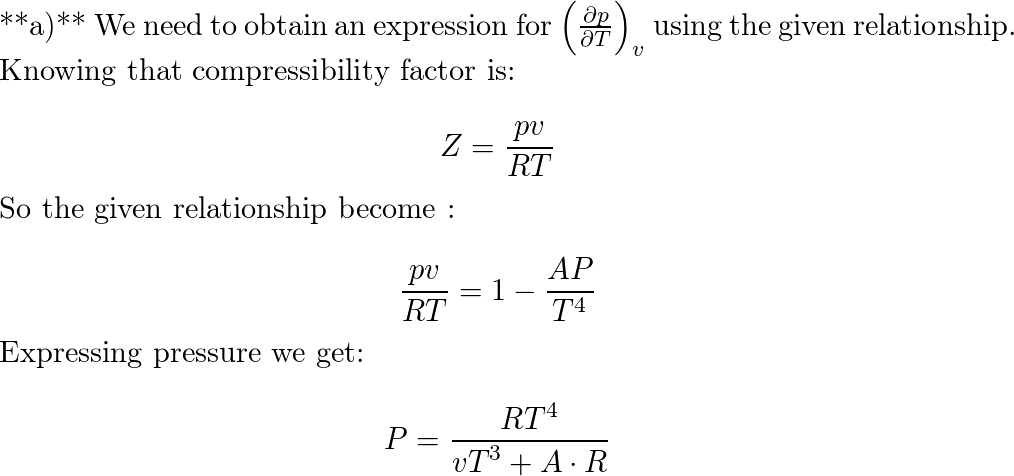At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to
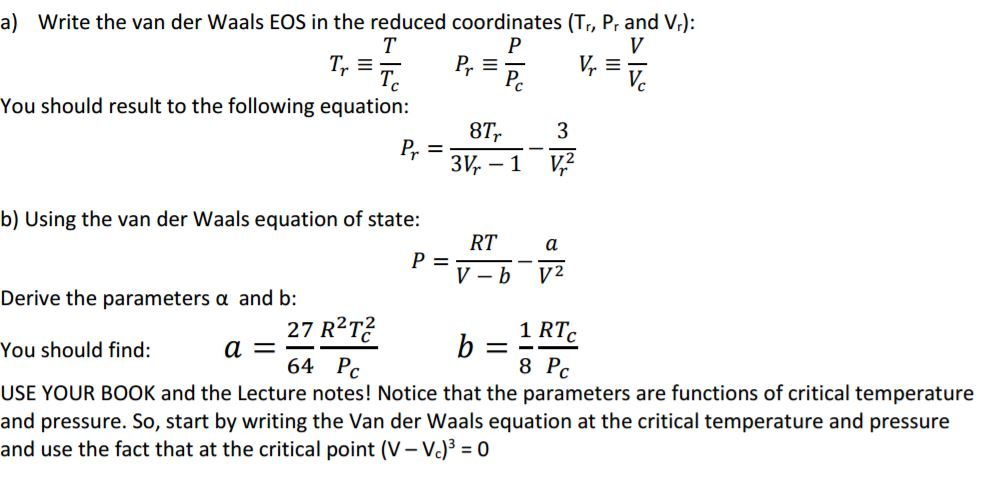
Solved Write the van der Waals EOS in the reduced

Van Der Waals Equation - an overview

MathType on X: Molecules in a real gas do interact with each
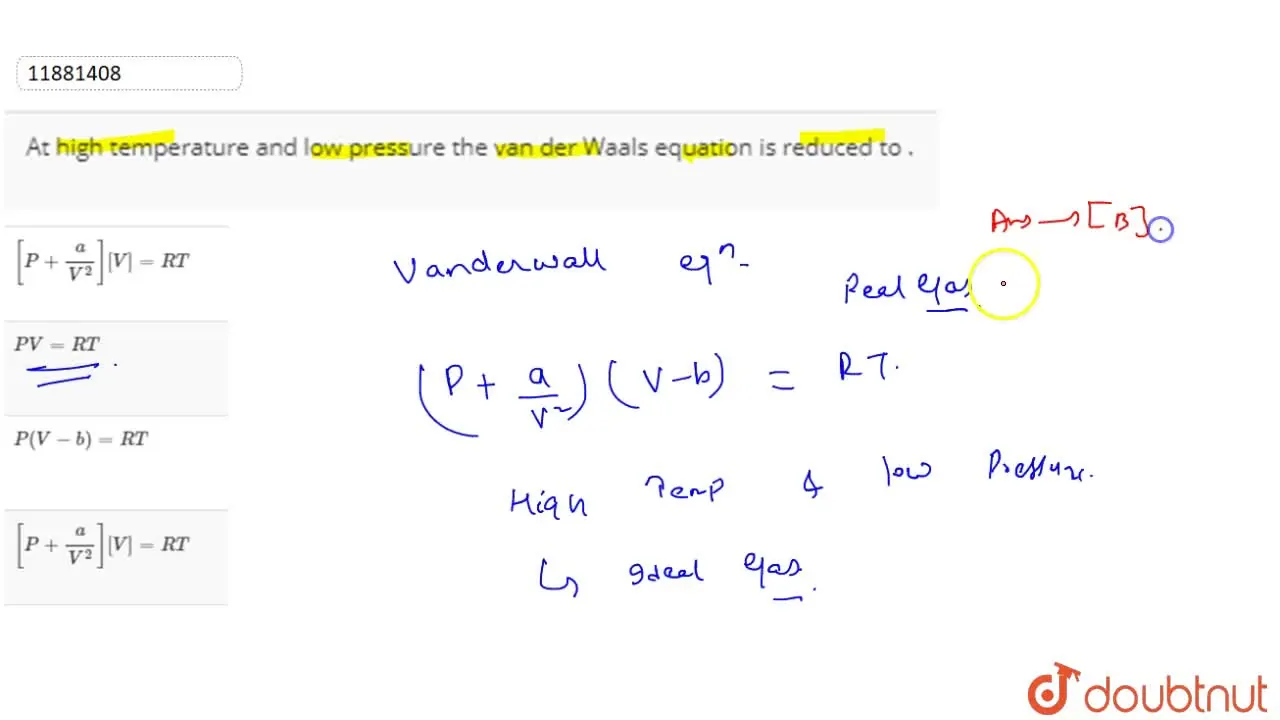
At high temperature and low pressure the van der Waals equation is red

Q.4 The van der Waals' equation for a gas is (p+V2a)(V−b)=nRT

Van der Waals equation - Wikipedia

Isotherms of van der Waals equation in reduced form, showing
p+a/v)(v b) =RT, p=pressure,v=volume,R,a,b are constant, T
How I find the a and b constant in the Van der Waals equation? - Quora

At low temperature if RT=2√ap , ( a is vander waal constant

The Van der Waals equation a real gas is : (P + 2) (V – b) = RT