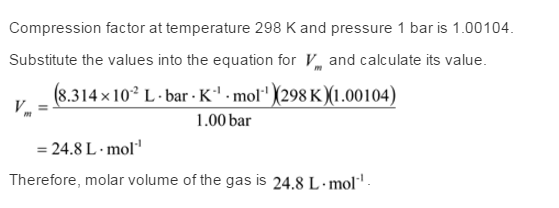
Show that the van der Waals equation leads to values of Z <

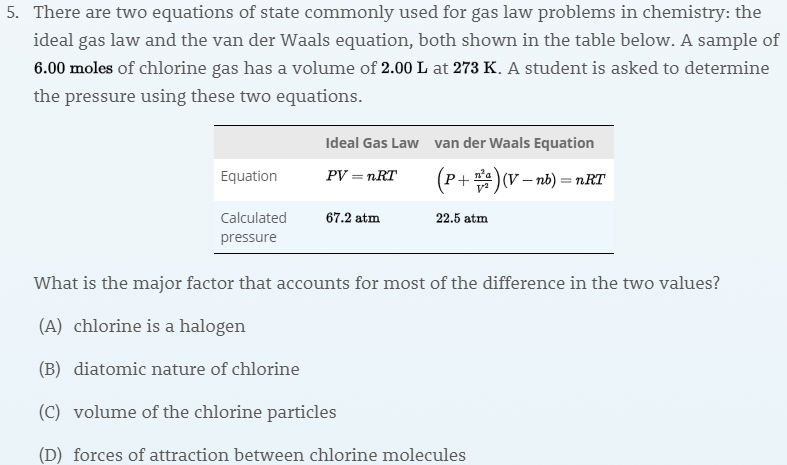
What is the major factor that accounts for most of the difference in these two values of pressure (ideal gas law vs. van der Waals equation)?

Chapter 1 Questions 8th Ed., PDF, Gases

Use the van der Waals equation and the ideal gas equation to calc

Assertion :Compressibility factor Z according to van der Waal's equation may be written as Z=cfrac {1}{1-(cfrac {nb}{V})}-cfrac {an}{RTV}. Reason: For real gases Z > < 1.Both Assertion and Reason are correct and

SOLVED: For a van der Waals gas with given values of a and b, identify the conditions for which Z < 1 and Z > 1.

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
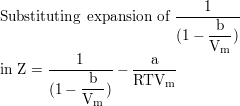
Show that the van der Waals equation leads to values of Z <
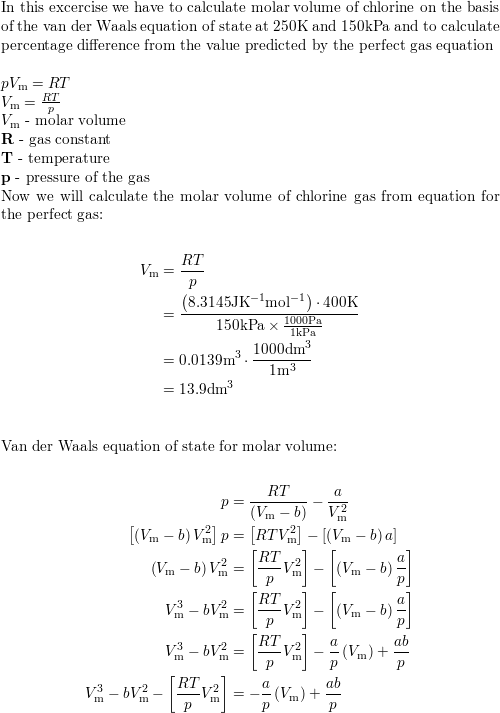
Calculate the molar volume of chlorine on the basis of the v

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very
Solved The formula for fugacity coefficient according to the

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Use the van der Waals equation and the ideal gas equation to calc
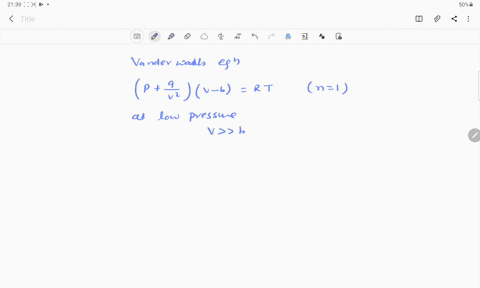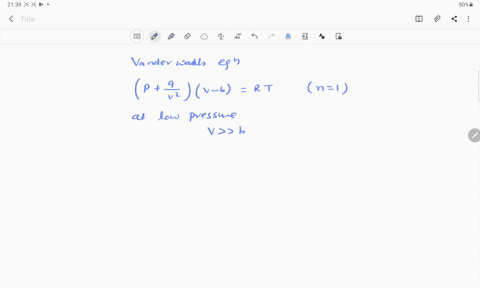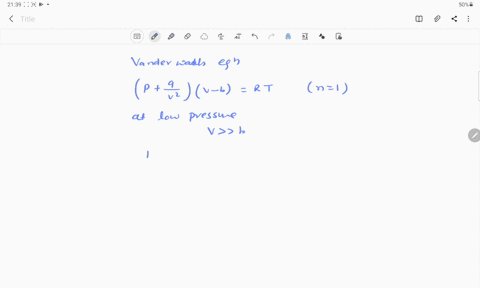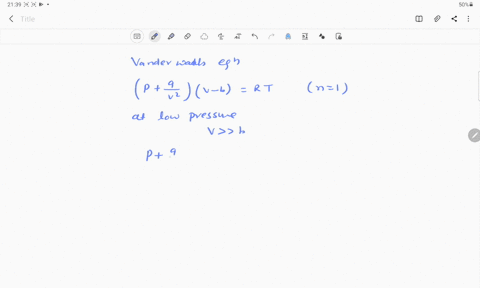
⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…
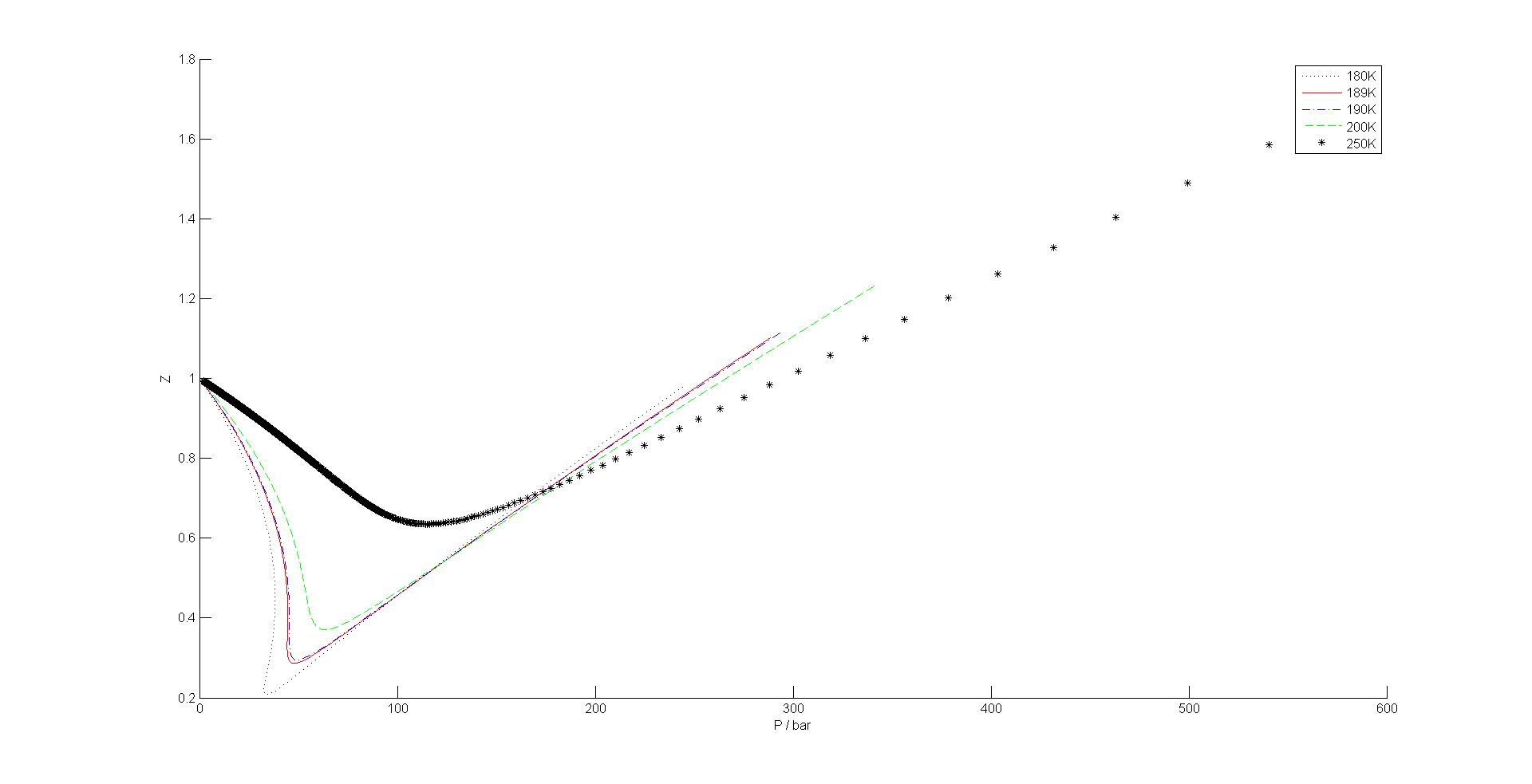
16.E: The Properties of Gases (Exercises) - Chemistry LibreTexts

Solved pPvdw= 4. Calculate the pressure of I mole of the