What is the change in internal energy (in J) of a system that

I found an increase of 3100J Have a look

What is the change in internal energy of a system which does 4.50x
CaptionSync Smart Player™

400 J of heat is added to a system. If the change in internal
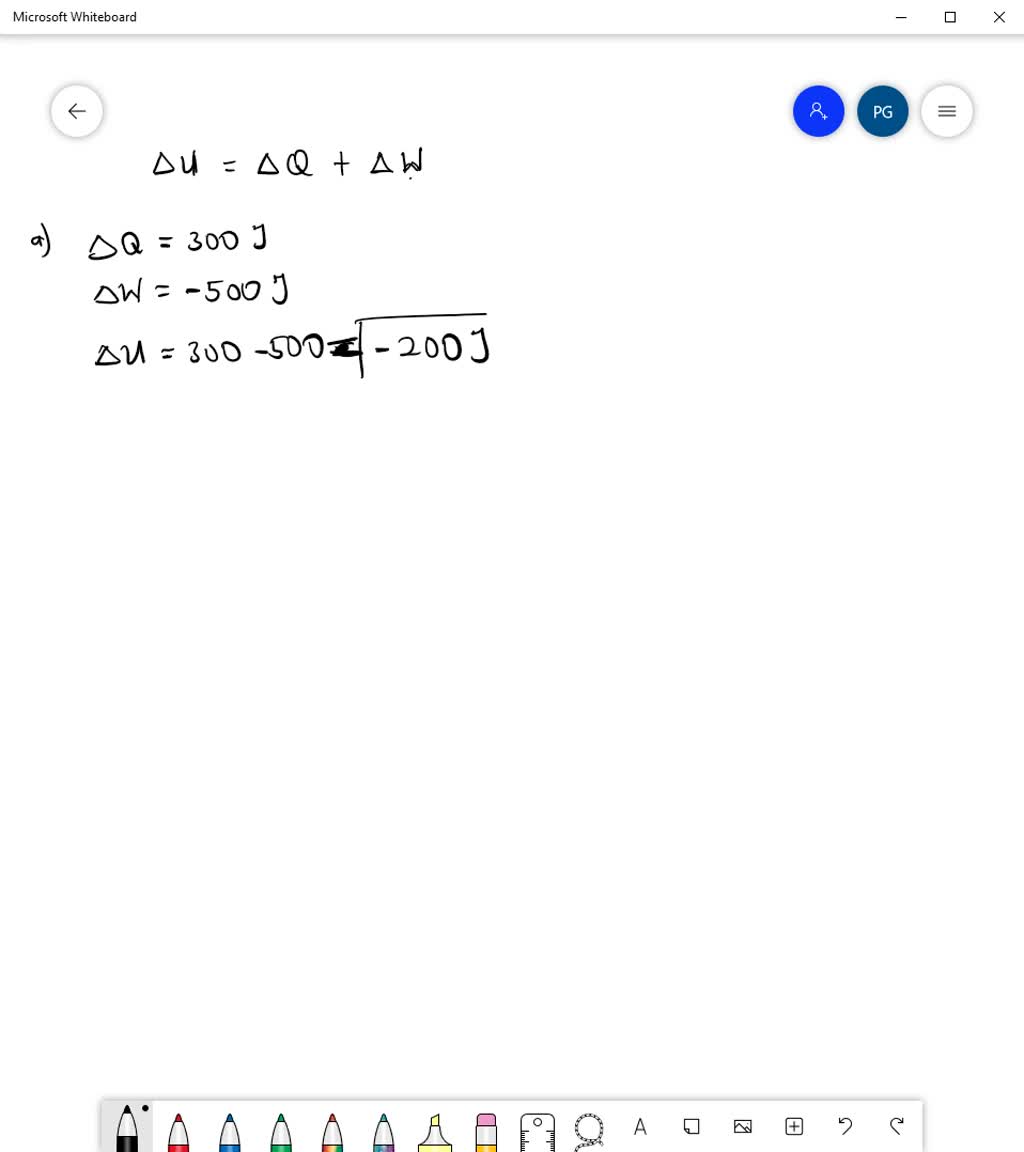
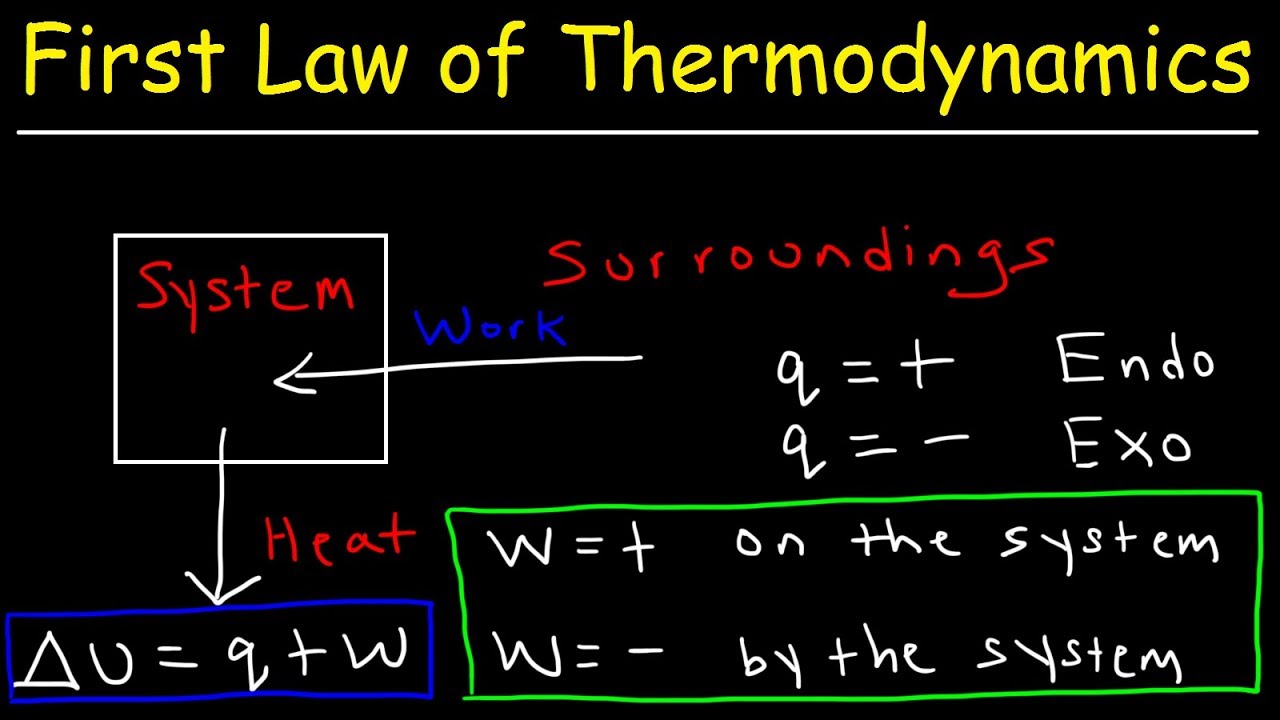
SOLVED: Calculate the change in internal energy. a) The system absorbs 300 J of heat and performs 500 J of work. b) The system absorbs 720 J of heat energy and the

Solved Be sure to answer all parts. What is the change in

500 J of heat was supplied to a system constant volume. It resulted in the increase of temperature of the system from 20^oC to 25^oC. What is the change in internal energy

Tutorial 2- Thermodynamic - CHM431 TUTORIAL 2 : THERMODYNAMIC SEM

Solved What is the change in internal energy (in J) of a
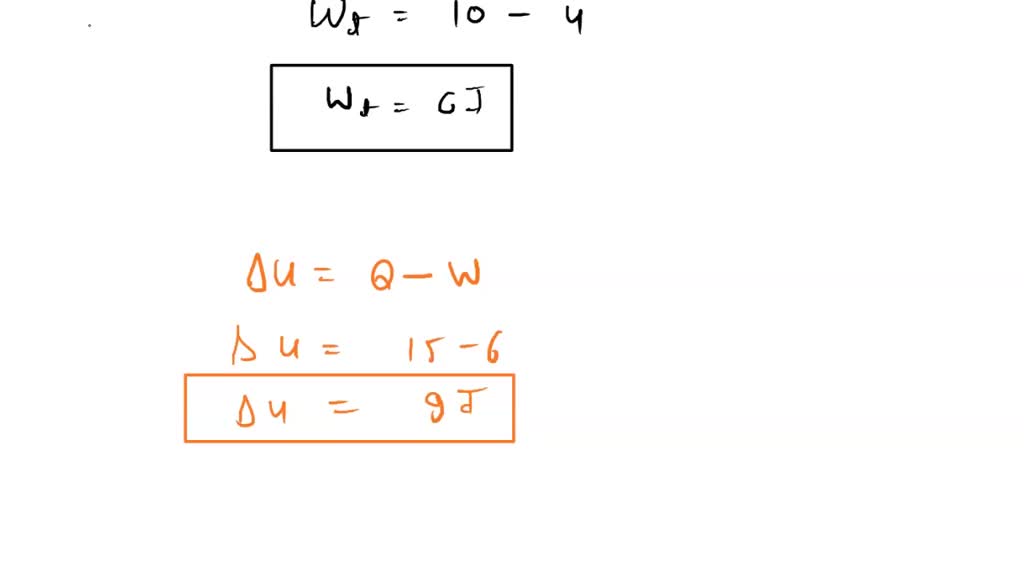
SOLVED: (a) Suppose there is heat transfer of 40.00 J to a system, while the system does 10.00 J of work. Later, there is heat transfer of 25.00 J out of the



:sharpen(0.5,0.5,true)/https://www.royce-lingerie.co.uk/static/media/catalog/product/1/0/1091_ls_maisie_navy.jpg)


