The vapour pressure of a solution having 2.0 g of solute X (gram
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

Understanding Liquid Solutions: Methods of Expressing Concentration, Vapour Pressure Concepts, and Raoult's Law, PDF, Solution

Vapor Pressure - Definition and How to Calculate It

14. The vapour pressure of the solution having 2.0 g of solute (a molecu the solution having 2.0 g of solute (a molecule of x with atomic ma = 32 g/mol) in
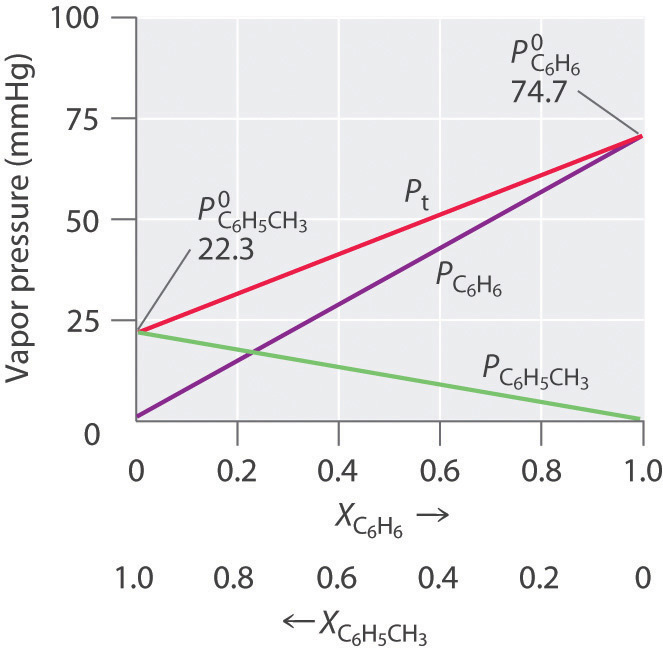
Colligative Properties of Solutions
When 2.5g of a non-volatile solute is added in 75g of water, the vapour pressure of the solution is 710mm of Hg. What will be the molar mass of the solute if
:max_bytes(150000):strip_icc()/mixed-race-scientist-lifting-beaker-in-laboratory-138711073-5c51bcdfc9e77c00013806d5.jpg)
Raoult's Law Example Problem - Vapor Pressure Change

SOLVED: The vapour pressure of a solution having 2.0 g of solute X

The vapour pressure of a solution having 2.0g of a solute X(molar mass 32gmol−1) in 100g of CS2 (vapour
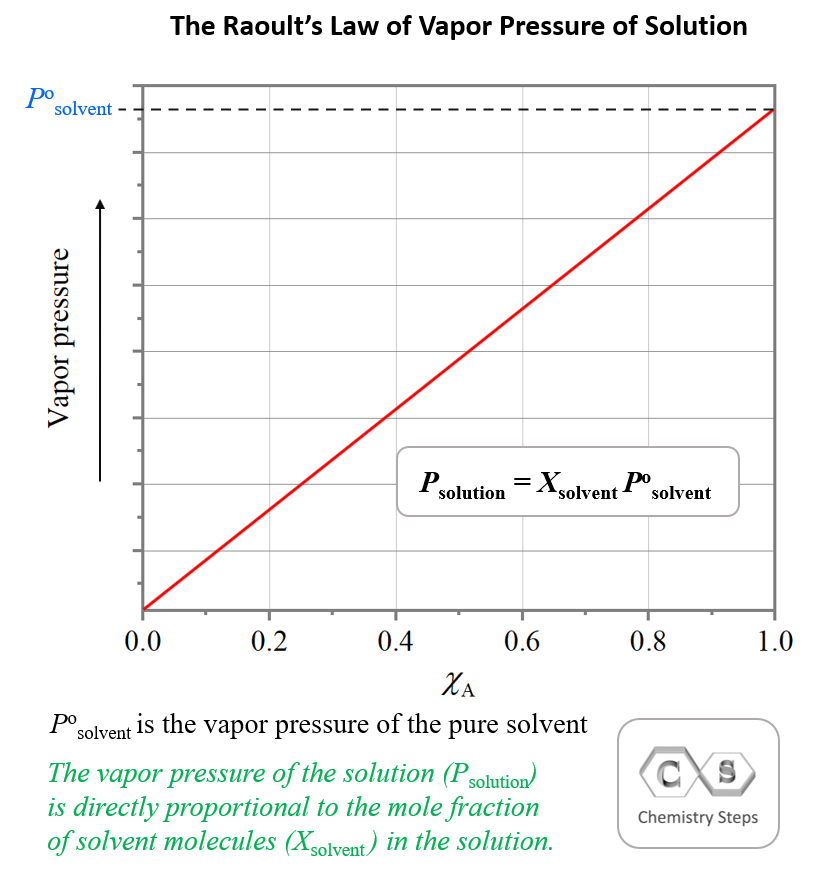
Vapor Pressure Lowering - Chemistry Steps

Calculate the mole fraction of toluene in the vapour phase which is in equilibrium with a solut

Omguloporou Wass 22. The vapour pressure of a solution having 2.0 o of solute X (gram atomic mass = 32 g mol-') in 100 of CS, (vapour pressure = 854 torr) is

Calculating Molar Mass Using Colligative Properties — Examples - Expii






