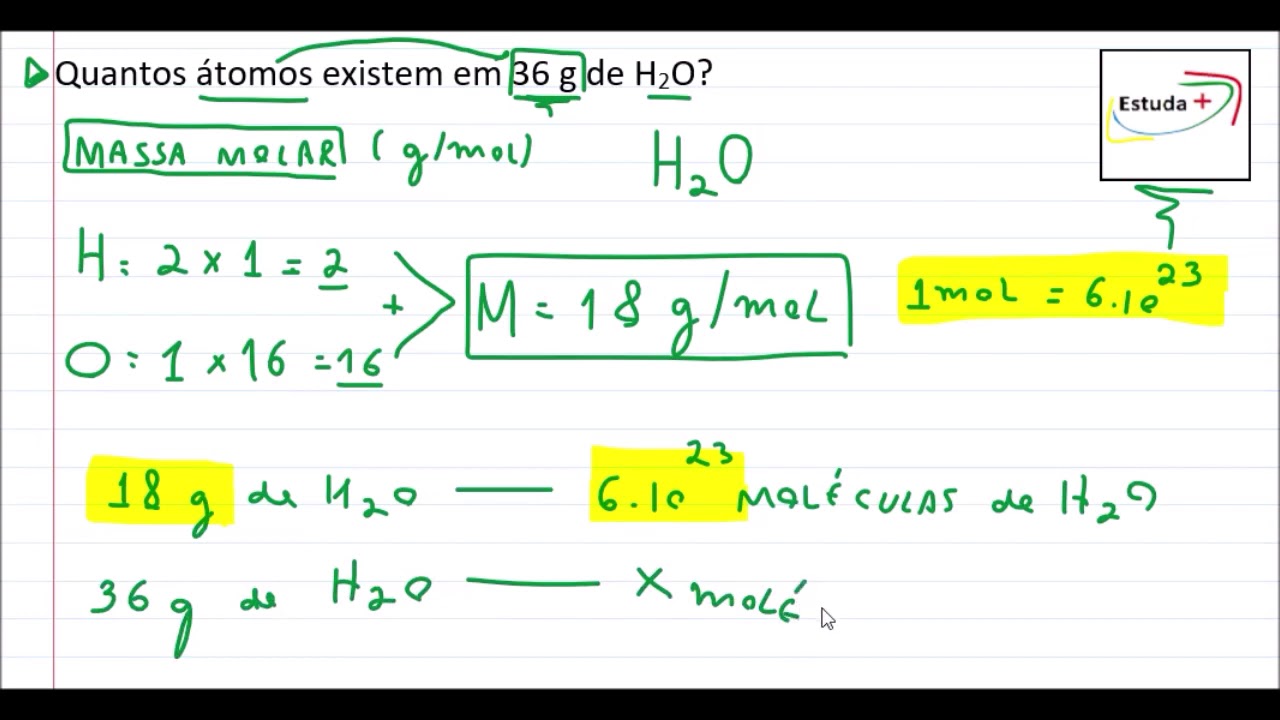
At 300 K, 36 g of glucose present per litre in its solution has an osm

pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

ANSWERED] elevation constant 18 The molar mass of a non volatile - Kunduz

What role does the molecular interaction play in a solution of alcohol

BIL360 DuBois Chapter 5: Transport of Solutes and Water Flashcards

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

At 300 K, 36 gof glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the same

Nutrients, Free Full-Text

Calculate the osmotic pressure of a solution containing 17.1 g of cane

State Henry's law and mention some important applications ?

B14. At 300k, 30g of glucose, C6H1206 present per litse en its solutior has an osmotic pressure of 4.98 bar. If the asmotic pressure of another glucose solution is 1.52 bar the

Kannada] At 300 K, 36 g of glucose present per litre in its solution

Polymers, Free Full-Text

ANSWERED] At 300 K 36 g of glucose present in a litre of its solution - Kunduz

12 At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressur of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars

A compound contains three elements X, Y and Z. The oxidation number of

At 300 K, 36 g of glucose present per litre in its solution has an osm