total number of atoms in 44 g of Carbon dioxide is ?
Total number of atoms in 44 g of Carbon dioxide is

oxygen atoms in 44g of carbon dioxide (atomic mass:C=12u,O=16u)
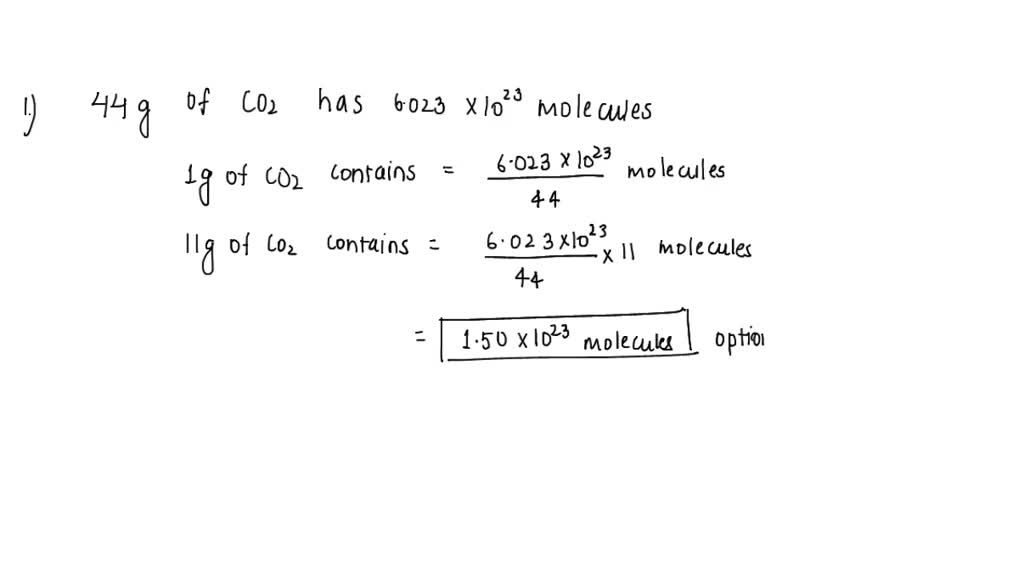
SOLVED: A 11.0 g sample of carbon dioxide (molar mass 44.0 g/mol). How many molecules does it contain? A. 6.02x10^23 B. 3.01x10^23 C. 1.50x10^23 D. 2.40x10^24 2. What is the mass of

Approach for C1 to C2 products commencing from carbon dioxide: A brief review - ScienceDirect

SOLVED: Assertion: Both 16g of CH4 and 44g of CO2 have the same number of atoms. Reason: Both contain 1g atom of carbon.

The total number of electrons in one molecular of carbon dioxide is
Sustainability, Free Full-Text
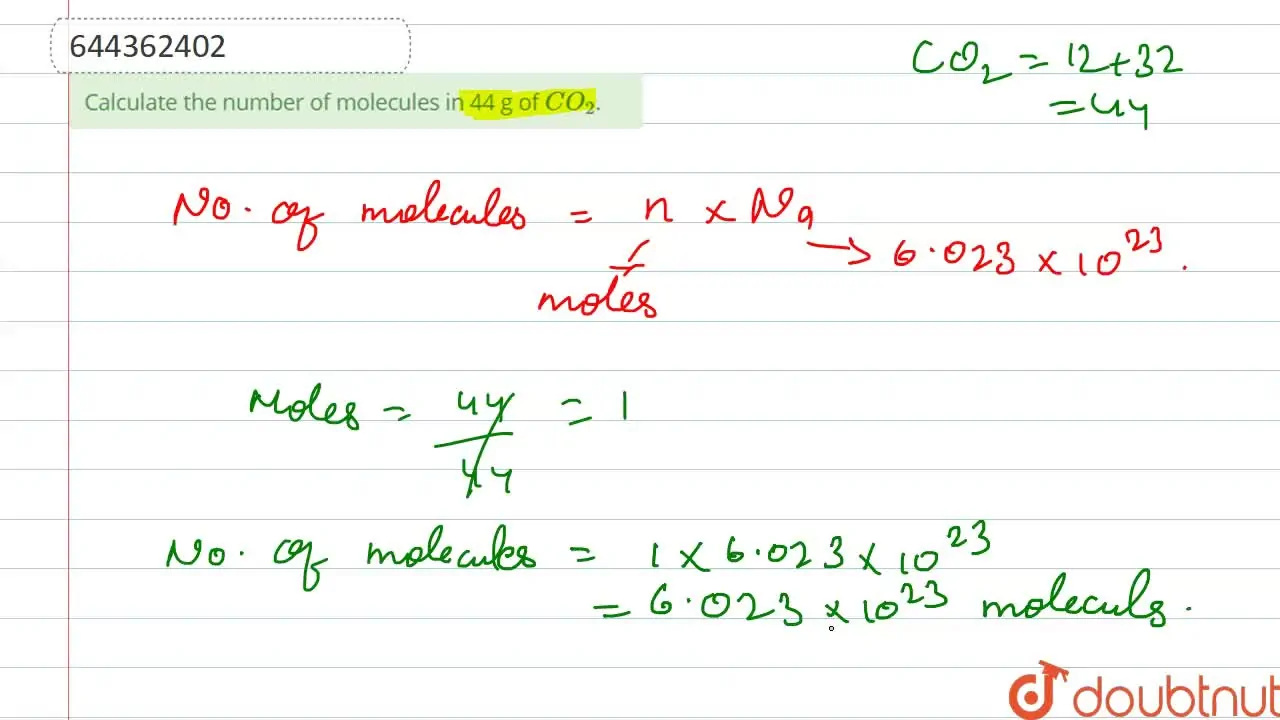
Calculate the number of molecules in 44 g of CO2.

How many atoms of oxygen are contained in 44g of CO2? - Quora

SOLVED: calculate the total number of atoms present in 44 gram of carbon dioxide
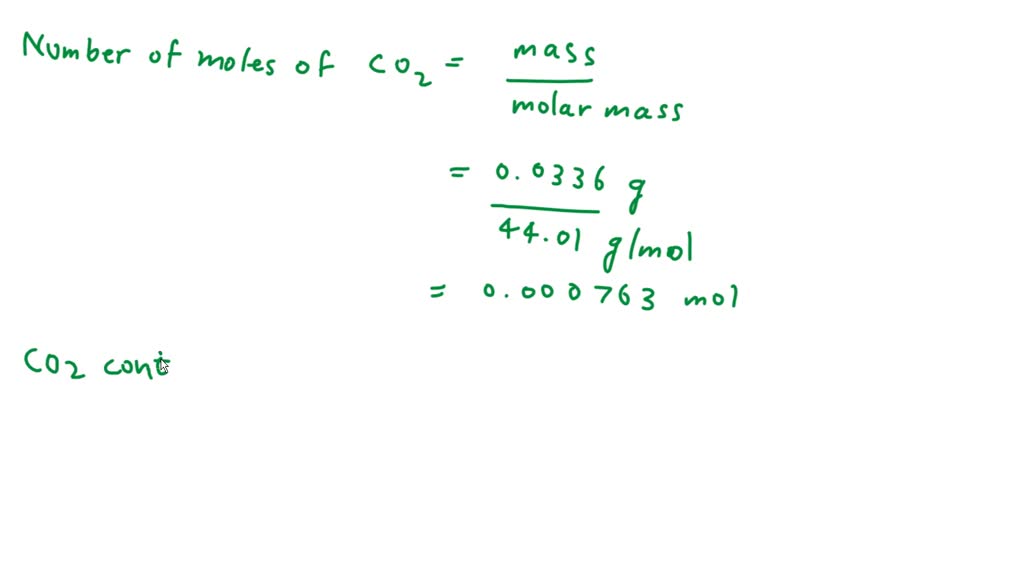
SOLVED: Atoms in 0.0336 g of CO2. The molar mass of CO2 is 44.01 g/mol. Find the number of moles of oxygen.
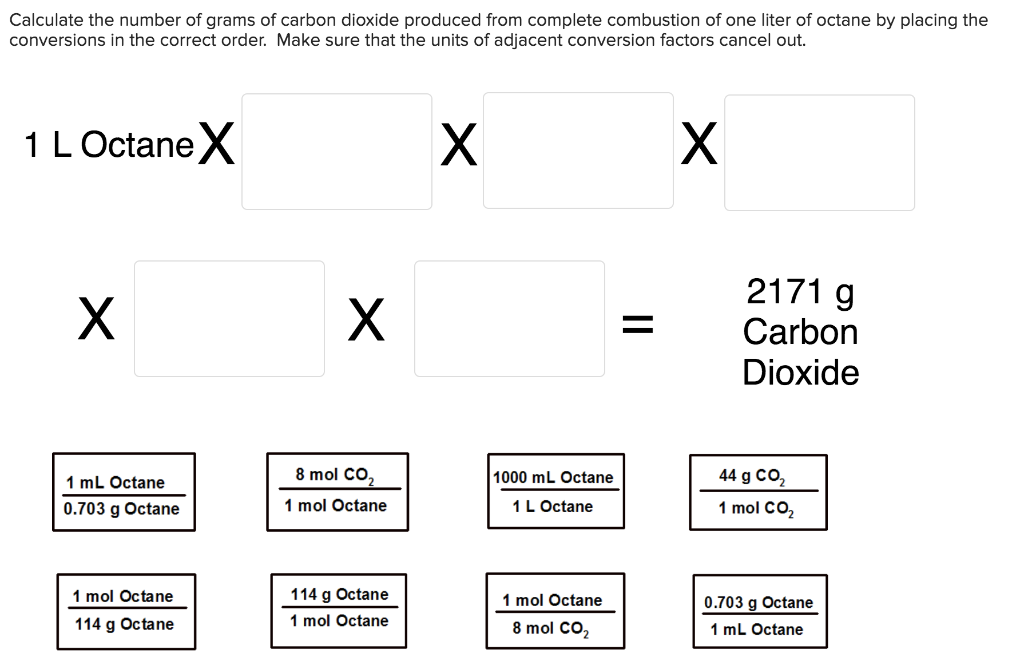
Solved Calculate the number of grams of carbon dioxide
How many atoms are present in 44 gm of CO2? - Quora