Solved ion of an ideal gas. The equation PI = constant (1)
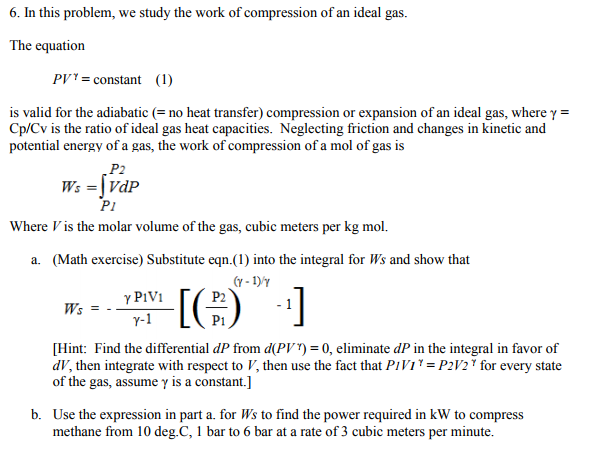
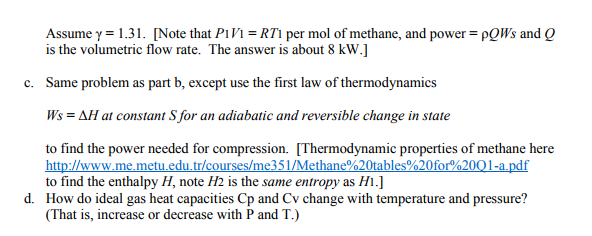
Solved ion of an ideal gas. The equation PI = constant (1)
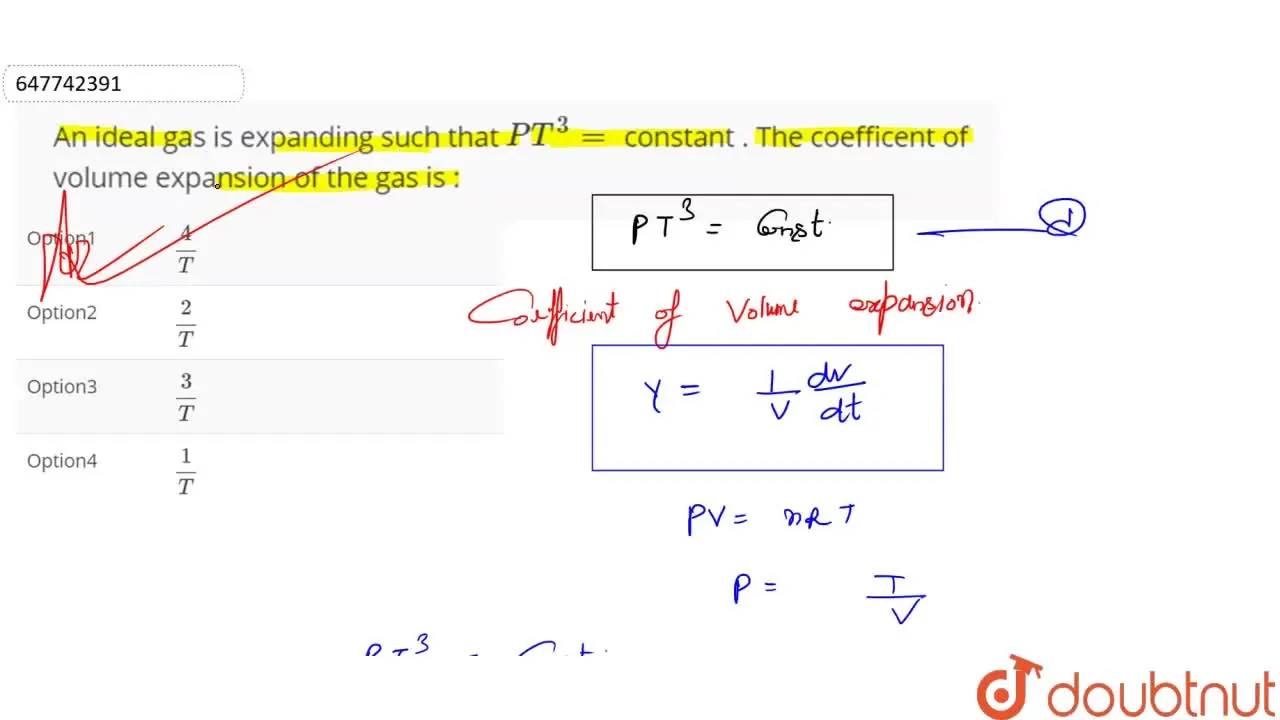
An ideal gas is expanding such that PT^(3)= constant . The coefficent

A non-ideal solution theory for the mechanics and electrochemistry of charged membranes
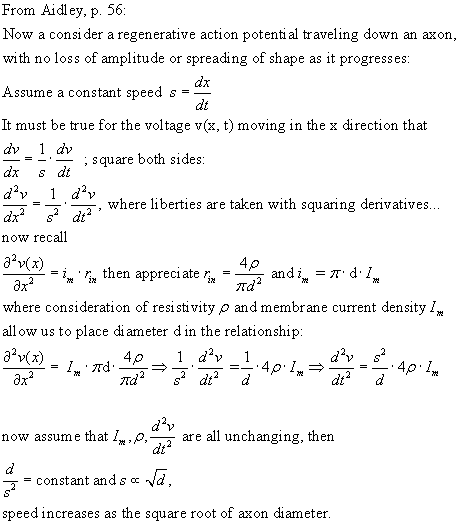
Nernst potential; conduction velocity
How to calculate the activity coefficient and the resulting activities of a mixture of 0.001M NaCl with 0.05 NaNO2 using Debye-Hückel approximation? Is the resulting activity coefficient only for one ion species
The Ideal Gas Law - Video Tutorials & Practice Problems

Use the van der Waals equation and the ideal gas equation to calc

achieve week 5 and 6 question 5 - CHEMISTRY COMMUNITY

SOLVED: ) One mole of an ideal gas expands from 5 to 1 bar at 298 K. Calculate w (a) for a reversible expansion and (b) for an expansion against a constant
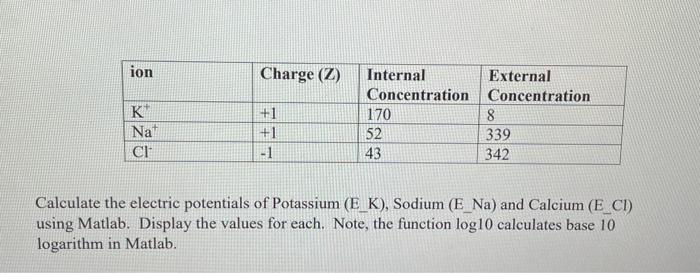
Solved In electrochemistry, the electric potential of an ion

PChem Quiz #1 Flashcards

SOLVED: [15 marks total] A cylinder of 12.0 moles of a diatomic ideal gas is in the following initial state: Pi = 25.0 atm, Ti = 293.0 K, V = 11.54 L.