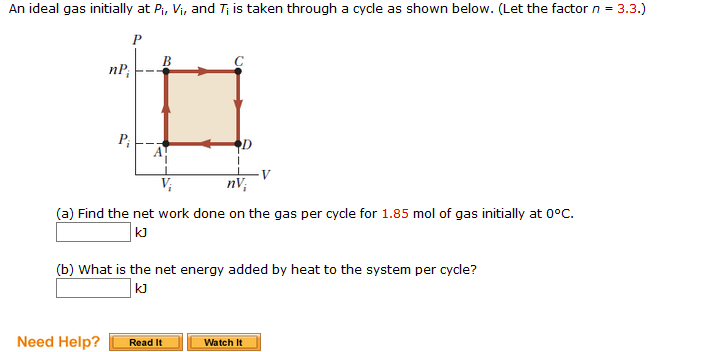
Solved An ideal gas initially at Pi, Vi, and Ti is taken

In the given figure an ideal gas changes its state from `A` to state `C` by two paths `ABC` and

First Alert Radon Gas Test Kit, RD1

Joule expansion - Wikipedia

Can AI Solve Science?—Stephen Wolfram Writings
Solved An ideal gas initially at Pi,Vi, and Ti is taken

Combined Gas Law — Overview & Calculations - Expii

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown in the figure (Pressure in Pa and Volume in m^3) What is the net energy

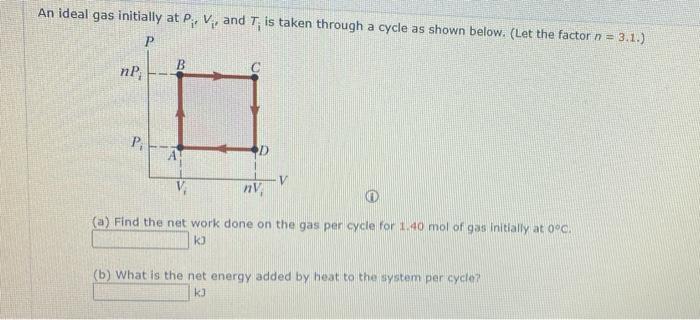
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n 3.6.) nP; P; nV; (a Find the net work done

1 mole of an ideal gas undergoes reversible isothermal expansion from an initial volume V_{1} to a final volume 10V_{1} and does 10 KJ of work. The initial pressure was 1times 10^{7}PaCalculate V_{1}
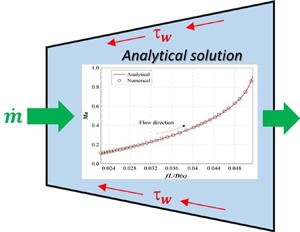
Exact solutions for quasi-one-dimensional compressible viscous flows in conical nozzles, Journal of Fluid Mechanics

SOLVED: Diatomic ideal gas (γ = 1.40) confined to a cylinder through a closed cycle. Initially, the gas is at Pi, Vir, and Ti. First, its pressure doubles under constant volume. It
Osmosis
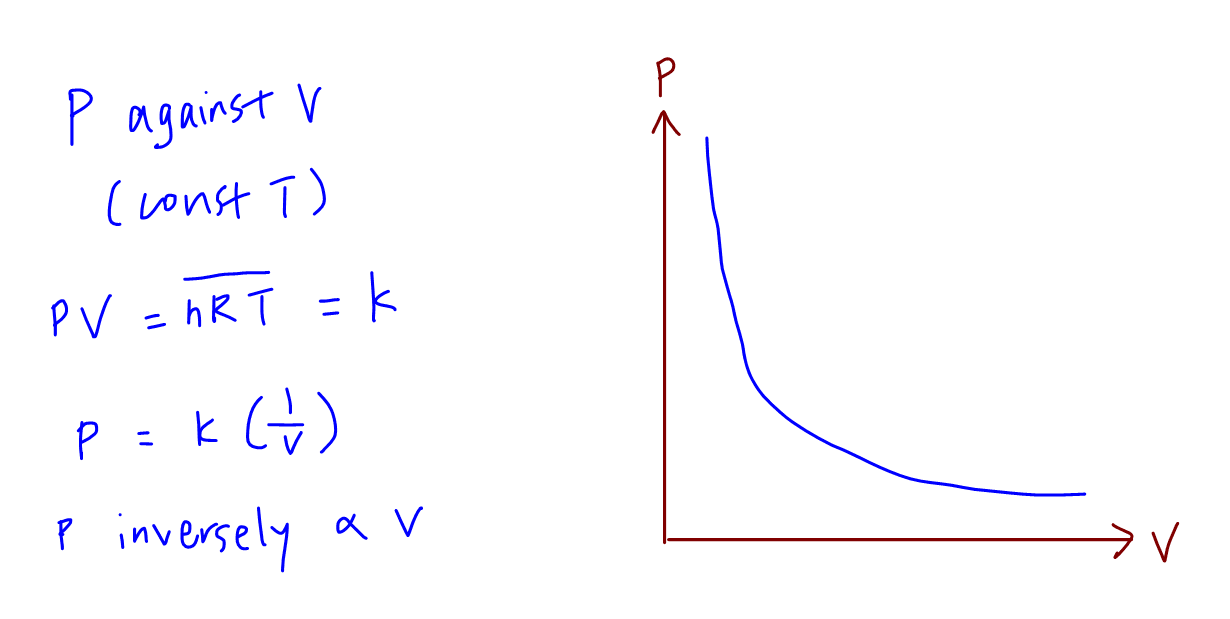
Ideal Gas Graph Sketching
Processes, Free Full-Text







