The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals
Is there a set of conditions at which the compression factor
Using van der Waals' equation, calculate the constant 'a' when two moles of a gas confined - Sarthaks eConnect

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
The compressibility factor for a real gas at high pressure is - Sarthaks eConnect

If Z is a compressibility factor, van der Waals equation at low pressure ..

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible, calculate the van der Waals' constant 'a' Domeik
The density of the vapour of a substance at 1 atm pressure and 500 K is 0.36 kg m^-3. - Sarthaks eConnect

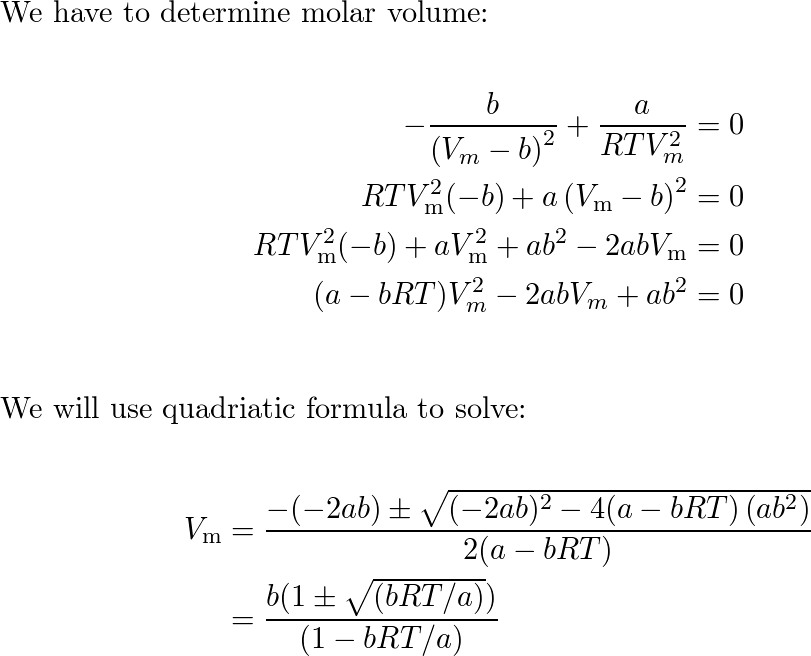
Solved Show that the compressibility factor of van der Waals

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible