42g of N₂ react with excess of O₂ to produce NO. Amount of NO

Share your videos with friends, family, and the world
16433-96-8, 1-Ethynyl-2-nitrobenzene
7693-52-9, 4-Bromo-2-nitrophenol
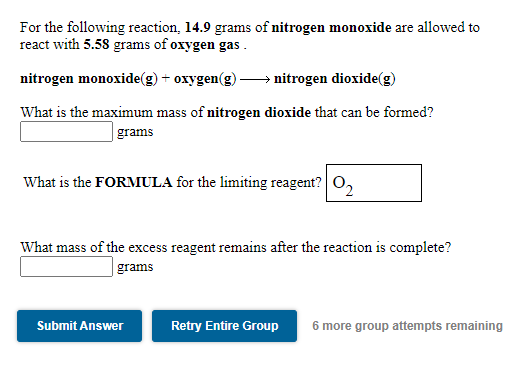
Solved For the following reaction. 14.9 grams of nitrogen
How many moles of N2 are needed to produce 42.4 moles NH3? - Quora
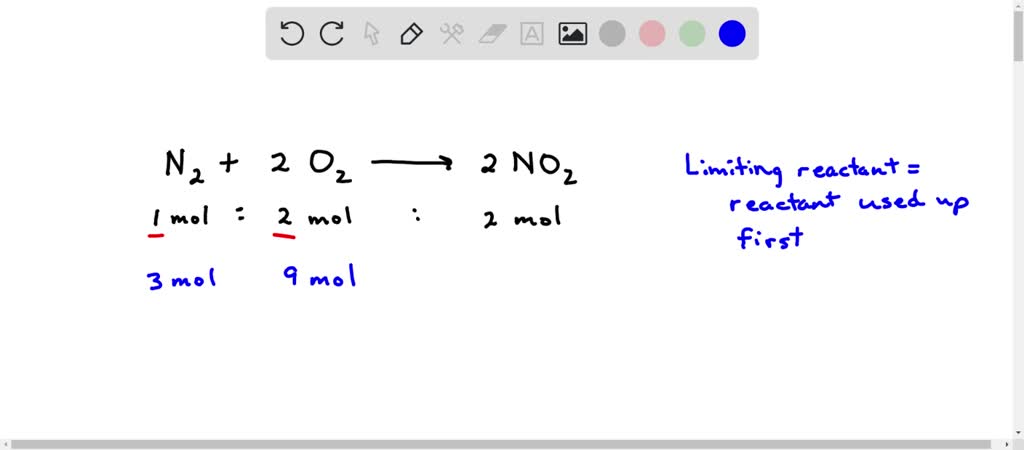
SOLVED: N2(g) + 2O2(g) â†' 2NO2(g). What is the limiting reactant when 3 moles of N2 and 9 moles of O2 react, and how much of the excess reactant remains after the

Answered: Consider the balanced reaction of…

Limiting Reaction Calculations Practice Flashcards

stoy-key-ahm-e-tree) - ppt download

KCSE-FORM-4-CHEMISTRY-NOTES (1) - Flipbook by nyangigerange04

Consider the reaction between NO(g) and O2(g) represented below. What is the balanced equation for this reaction and what is the limiting reactant?
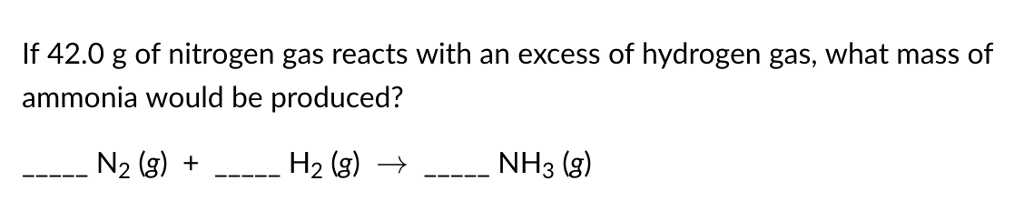
Solved If 42.0 g of nitrogen gas reacts with an excess of

UMAIR KHAN ACADEMY







