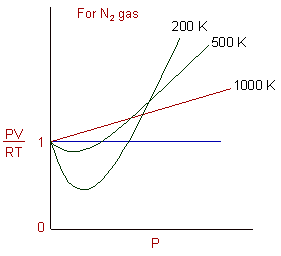
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Assignment gaseous state_jh_sir-2621

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Properties of gases extended oct 2020

The compressibility factor of a gas is defined as Z=PV/nRT. The compressibility factor of an ideal gas is:1-1zeroinfinite

Real Gas Behavior The Compression Factor (Z) [Example #2]
Essential Pharma Documents: 1205: Properties of Gases

Gas compressibility factor Z: Ideal gas vs Real gas

Chapter03.pure substance

Properties of Gases & Gas Mixtures, PDF, Gases

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `




:format(webp)/https://static-sg.zacdn.com/p/sunnydaysweety-0997-8848721-2.jpg)
