4. A container contains 32 g of O2 at a temperature TThe pressure
4. A container contains 32 g of O2 at a temperature TThe pressure of the gas is P. An identical containercontaining 4 g of H2 at a temperature 2T has apressure of(1) 8P(3) P(2) 4P(4) P18r cnstant
4- A container contains 32 g of O2 at a temperature TThe pressure of the gas is P- An identical containercontaining 4 g of H2 at a temperature 2T has apressure of-1- 8P-3- P-2- 4P-4- P18r-cnstant

Solved (7) Consider the following statements: Consider the

toppr-doubts-media.s3.aws.com/images/1948236
A vessel has 6g of oxygen at a pressure P and temperature 400 k. A small hole is made in it so that O2 leaks out. How much O2 leaks out if

A container holds 1.0 g of oxygen at a pressure of 8.0 atm.
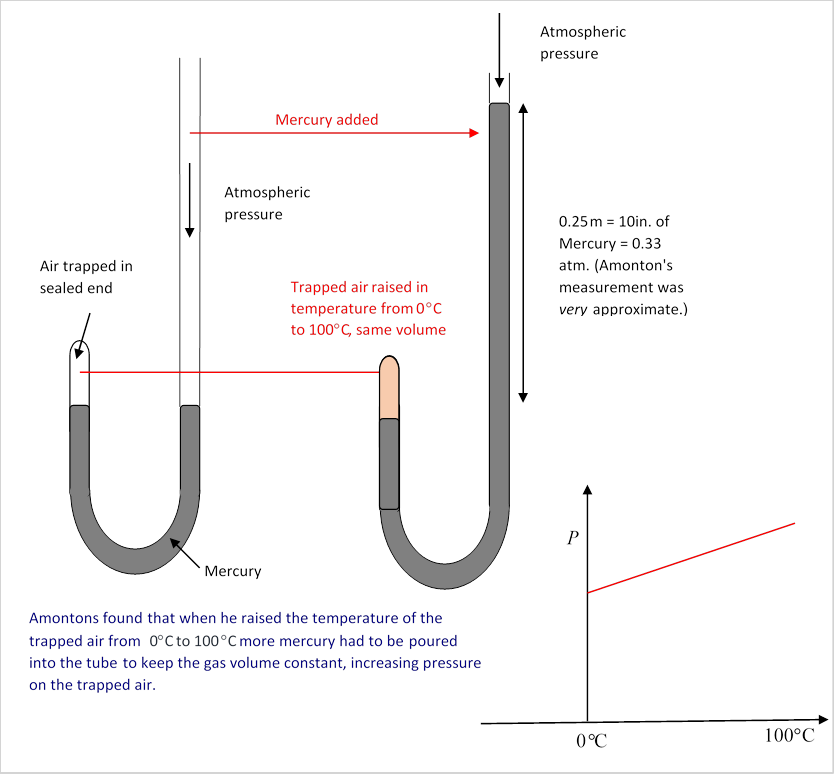
Gas Law and Avogadro

A sealed container contains a mixture of oxygen and nitrogen gas.The ratio is .The ratio is

8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

toppr-doubts-media.s3.aws.com/images/1818262

A container contains 32 g of O, a temperature T. The pressure of the gas is P. An identical container containing 4 g of H, a temperature 2T has a pressure of (

A container of 5 i has a gas p=0.87 mathrm{m} of mathrm{Hg}_{32} tols is joint to an evaguent conlainere 3,-frac{331}{0.8} mathrm{m} capiaty. The rementing pressuren p=8 mathrm{g} mathrm{Hg} begin{aligned} r & text {
What is the limiting reagent in the reaction. S8 + 8 O2 - - - - - - > 8SO2. Where sulphur weighs 32g and oxygen also weighs 32 g? - Quora
The equilibrium constant (K) for the reaction,2SO2(g)+O2(g)2S03(g) at 1000 K is 3.5 atmWhat would be the partial pressure of oxygen gas,if the equilibrium is found to have equal moles ofSO2 and SO3?







