What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0


Elasticity, Strength, and Water Permeability of Bilayers that Contain Raft Microdomain-Forming Lipids: Biophysical Journal
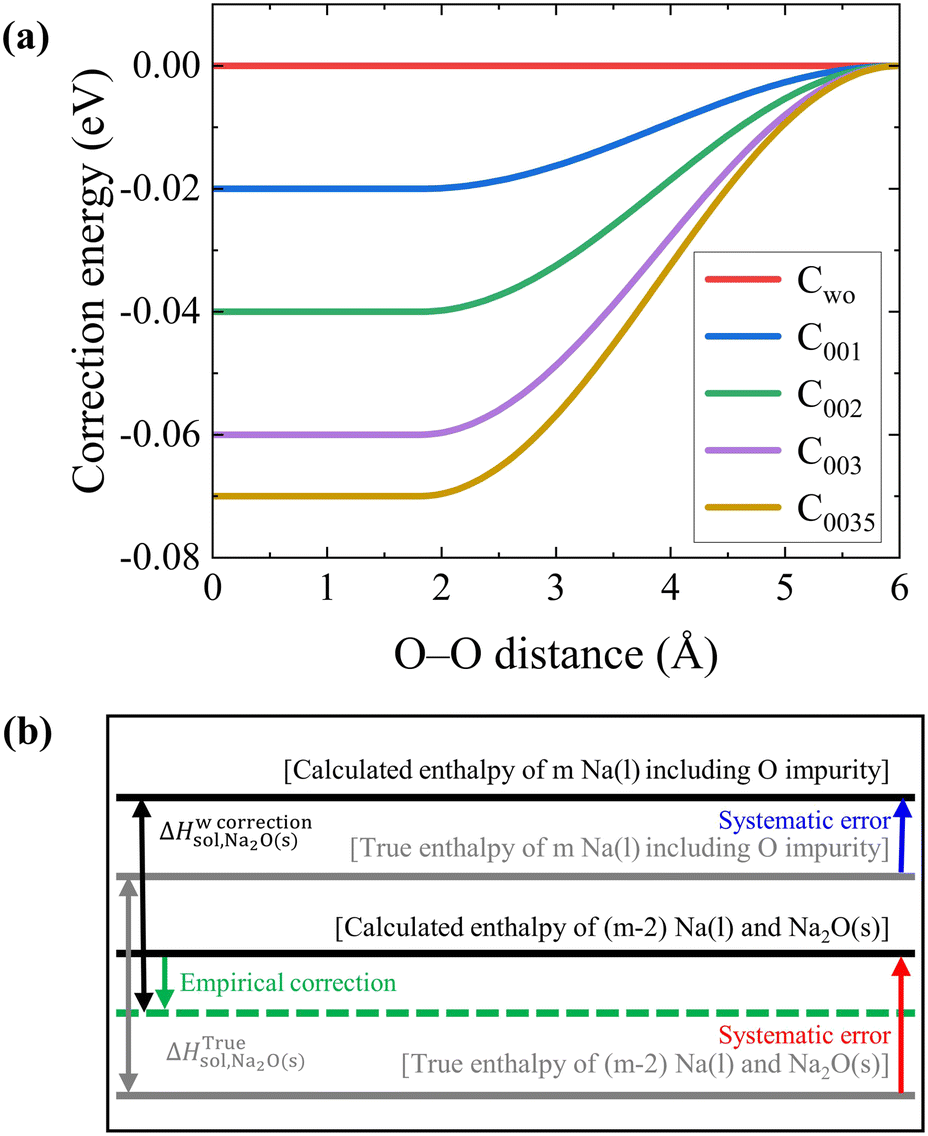
Temperature dependence of O solubility in liquid Na by atomistic simulation of Na(l)–Na 2 O(s) interfaces using corrected machine learning potential: - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D3CP01348K

Solved The van der Waals equation of state can be used to

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Real Gases Introductory Chemistry

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

Chemical Thermodynamics
The compression factor (compressibility factor) for 1 mol of a van der
.jpg?revision=1)
Gas Laws - Overview - Chemistry LibreTexts
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas
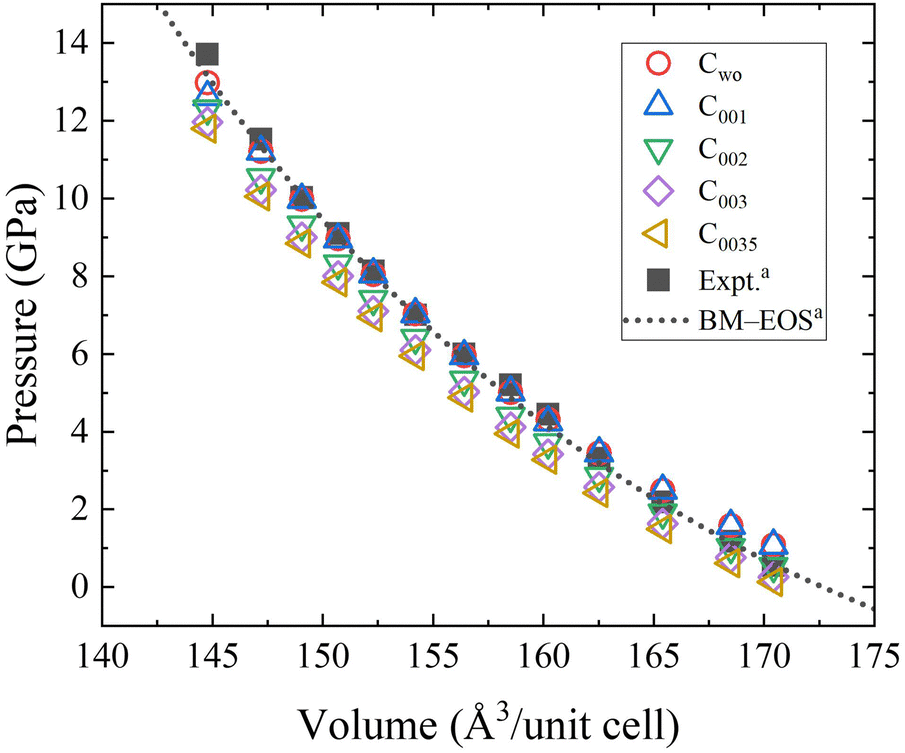
Temperature dependence of O solubility in liquid Na by atomistic simulation of Na(l)–Na 2 O(s) interfaces using corrected machine learning potential: - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D3CP01348K

PDF) New Correlation for Hydrogen-Natural Gas Mixture Compressibility Factor

The second virial coefficient obtained from different models for Nitrogen.

02 mole of a van der Waals gas pressure of 0.1 alin. Civanges unpredictably (B-16. What is the compressibility factor (Z) 0.02 mole of a Assume the size of gas molecules is
What is the compressibility factor (Z) for 0.02 mole of a van der Waal







