Consider the graph between compressibility factor Z and pressure P

Z1 means force of attraction dominating ie a is considerable b can be negligible at low temperature and low pressure Lower is the value of Z easier is the process of liquification
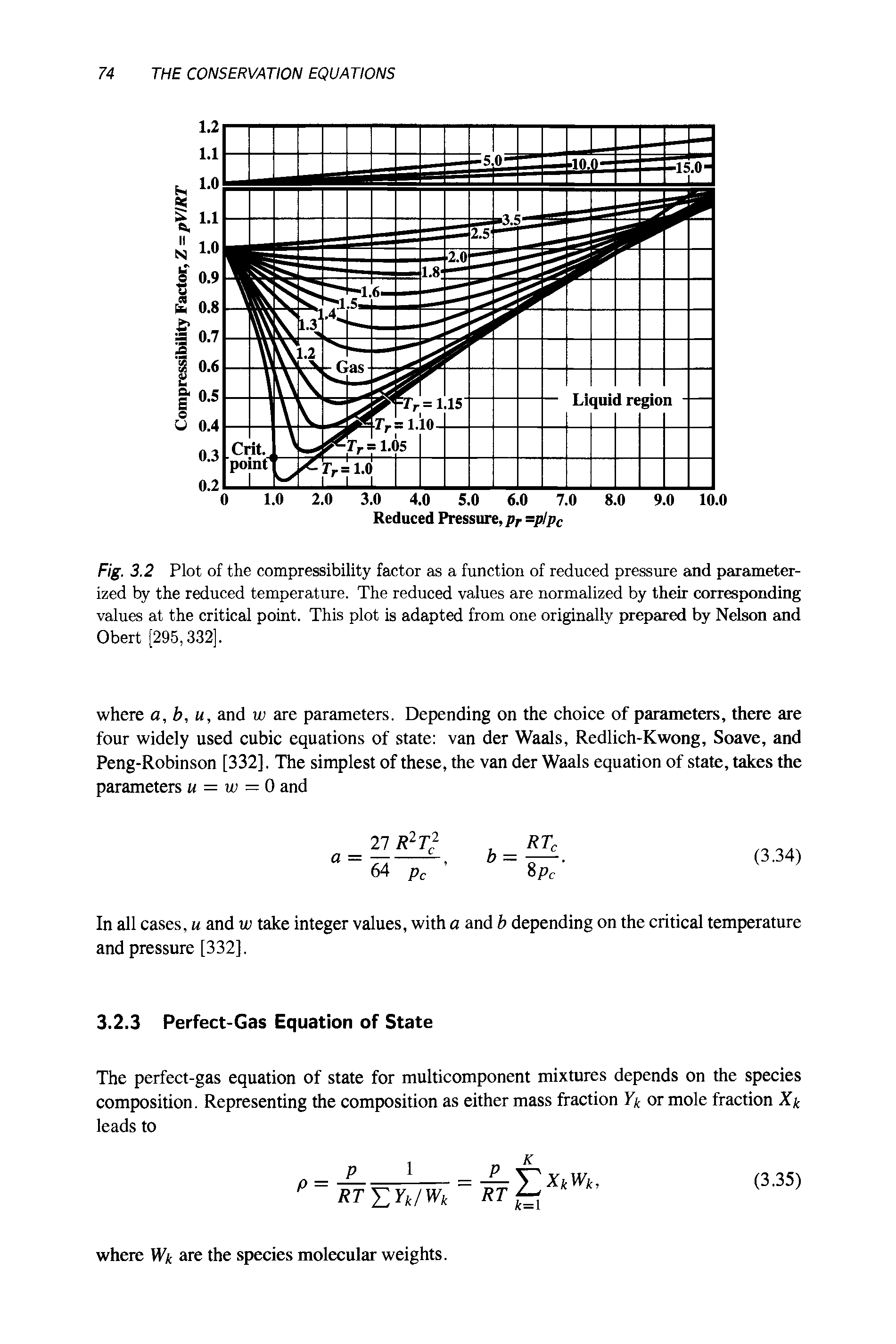
The compressibility factor is actually a factor that corrects the actual value of the gas versus the ideal gas. Let us learn and understand this concept.
Watch this video to understand the behaviour of real gases with the help of the compressibility factor. This is an important topic for JEE main.
What is the compressibility factor, and how does it vary with an increase in temperature and pressure? Watch this video to get the answer. This is an importa

Praveen-Fl (22-23) MCT - 1, PDF, Acceleration
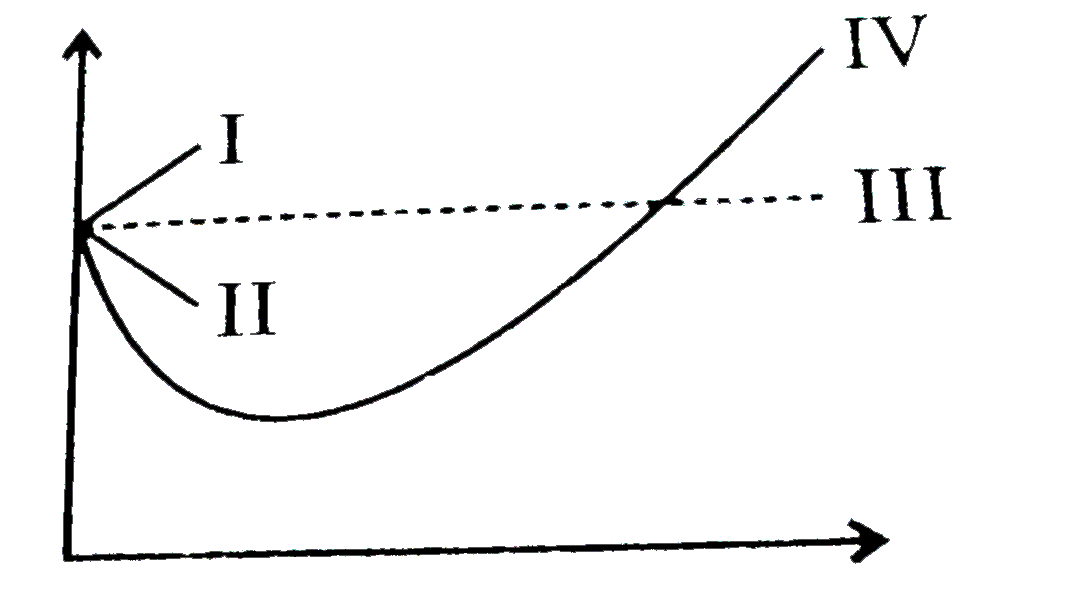
Telugu] The variation of compressibility factor (Z) with pressure (p
11.3: Critical Phenomena - Chemistry LibreTexts

Consider the graph between compressibility factor Z and pressure P
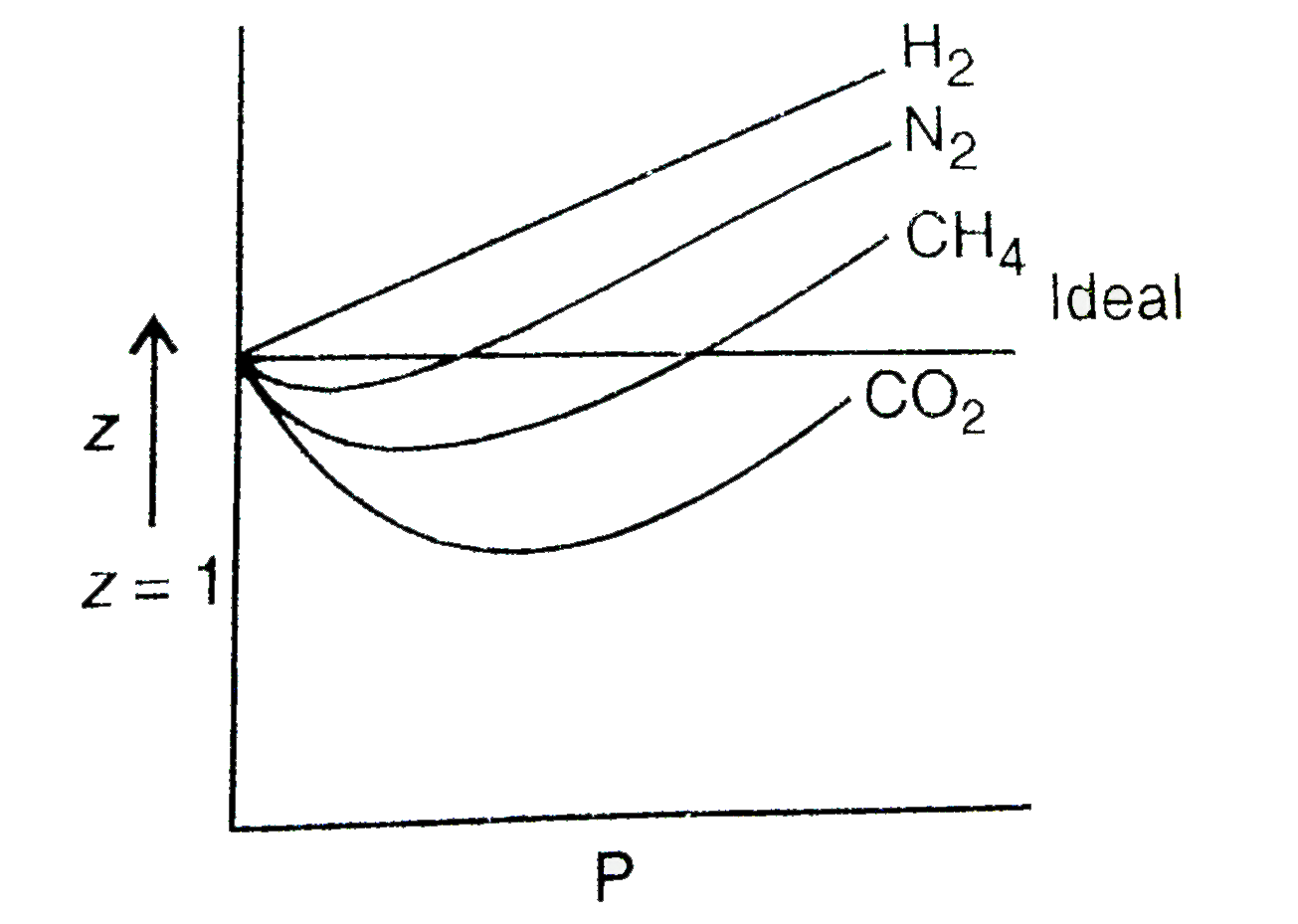
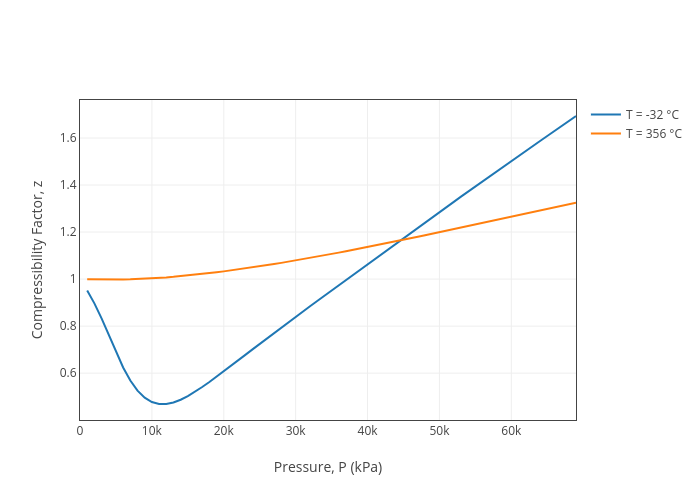
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

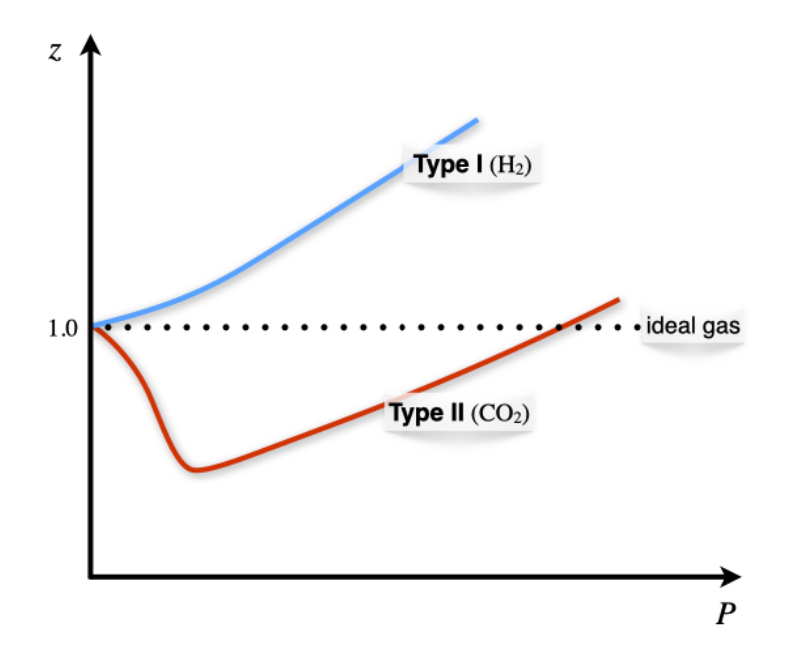
The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.
Standing and Kats Compressibility Factor Chart (Ahmed 2006)
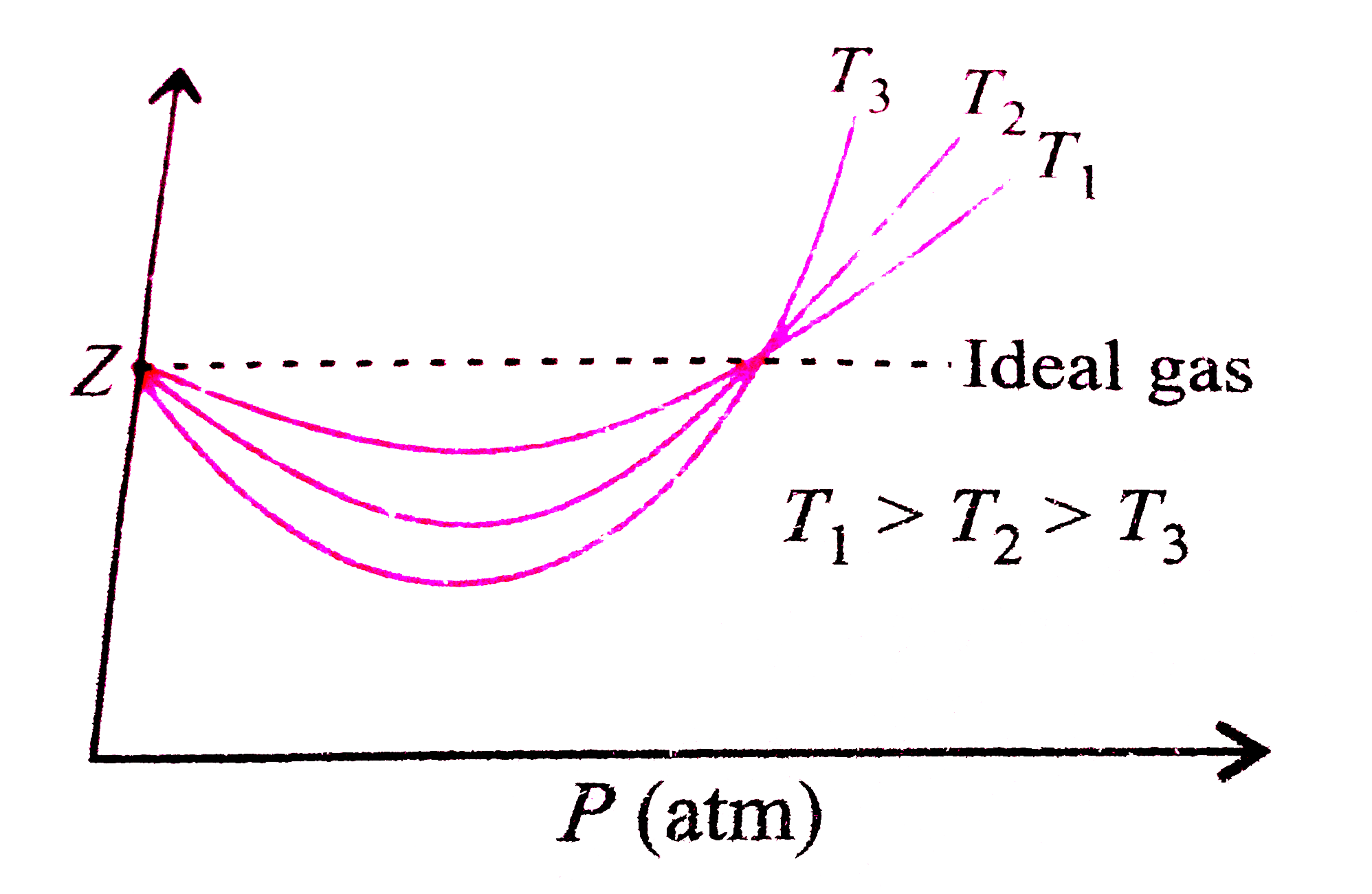
Consider a graph between compressibility factor Z and pressure P

pH of a 100 cc solution is 2. It will not change if
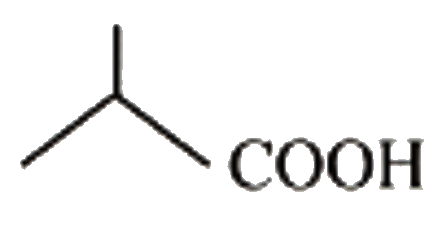
How many of the following acids will show higher reactivity towards es

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange