What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?
By A Mystery Man Writer

I found an increase of 3100J Have a look
A system absorbs 180 J of heat and does 160 J of work. What is the

Heat Transfer by J P Holmann

ME532 AdvancedHT IIConvectionandMassTransfer PDF, PDF

Punjabi] A system 500 j or heat and does work of 50 j on its sourroun

How to calculate ΔE when the system absorbs 250 J of heat energy

Charlotte Aaron Physics Tutor on HIX Tutor

The elastic properties, elastic models and elastic perspectives of
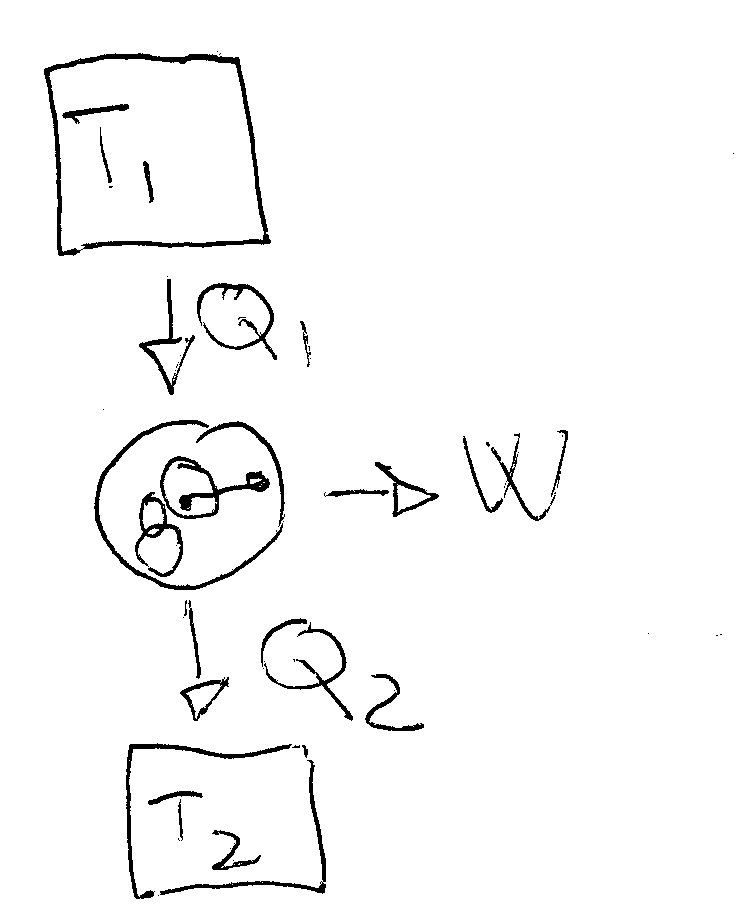
Heat and Work - Physics

Appendix CA: Modified National Standard for Buildings, Except for
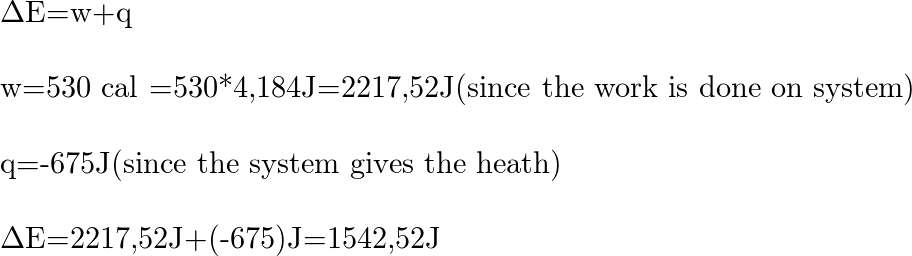
What is the change in internal energy (in J) of a system tha