20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

Solved Problem 2. ( 30 points) The ideal gas law can

Cubic equations of state - Wikipedia

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

Compressibility factor Z versus ρ ¯ for the n = 4 fluid. The plot

At low pressure the van der Waals' equation is reduced to [P +(a)/(V^(

If Z is a compressibility factor, van der Waals' equation at low pressure can be written as

Solved The ideal gas law can represent the

Solved Problem 1: Molar Volume and Compressibility Factor
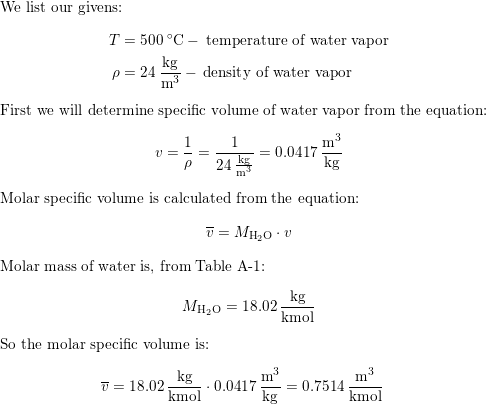
Estimate the pressure of water vapor at a temperature of $50
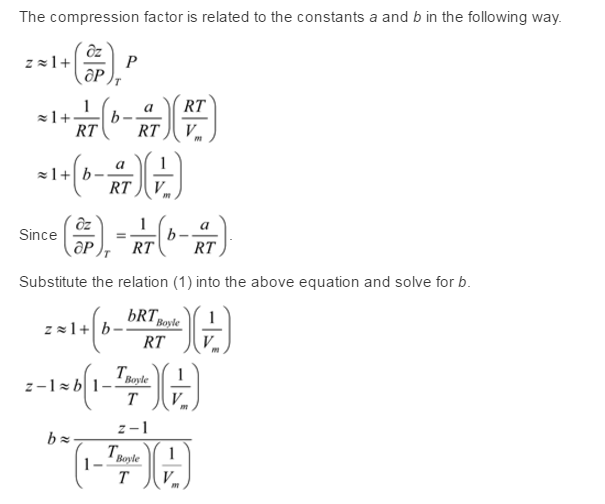
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)

At low pressure, the compressibility factor is given as

66. If z is the compressibility factor, van der Waals equation low pressure can be written as: (A) Z = 1 + PT (B) 2 = 1 - VT (C) 2=1 - (0) 2 =1+ PT Space rough use

1.7: Connecting the van der Waals and the viral equations- the Boyle temperature - Chemistry LibreTexts
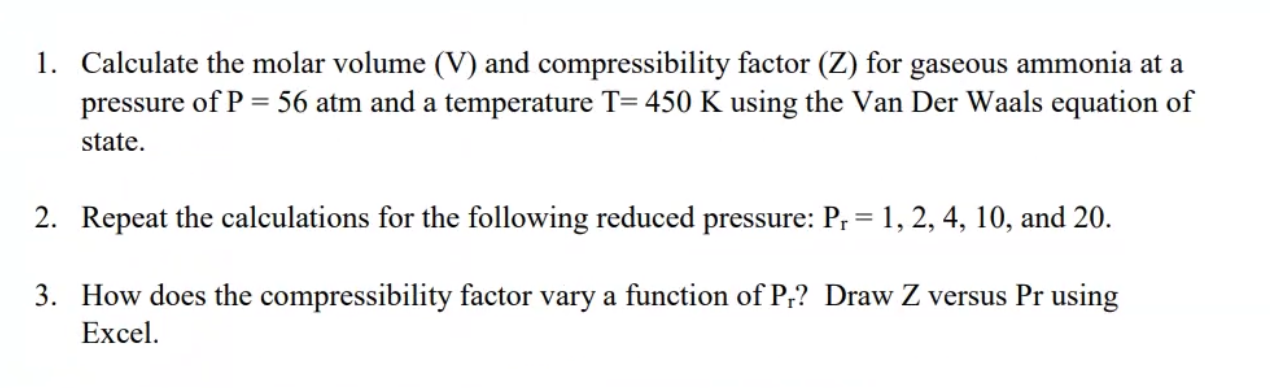
Solved The Van Der Waals equation of state is given by
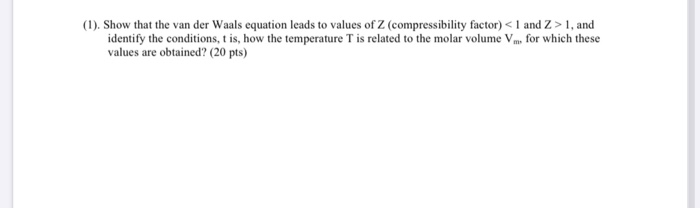
Solved (1). Show that the van der Waals equation leads to







