What is the value of compression factor Z for the gas? (A) 1 (B) >1 (C) <1 (D) Zero
By A Mystery Man Writer


ReasonAll the gases tend to approach a value Z=1, when the

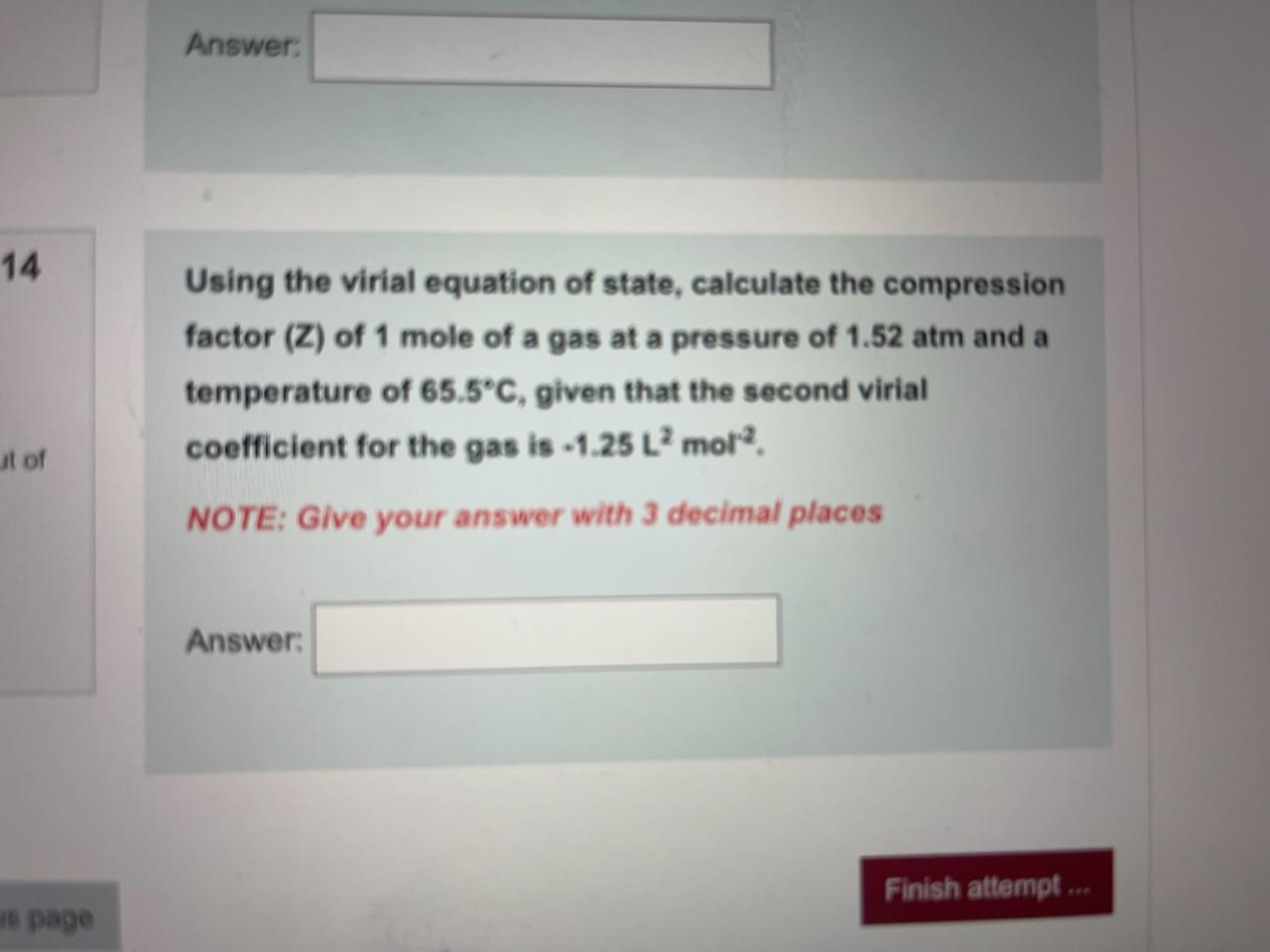
The compressibility factor for a real gas is expressed by, z =1+ BP / RT. The value of B at 500 K and 600 bar is 0.0169 L / mol. Find the

Compression Factor Z
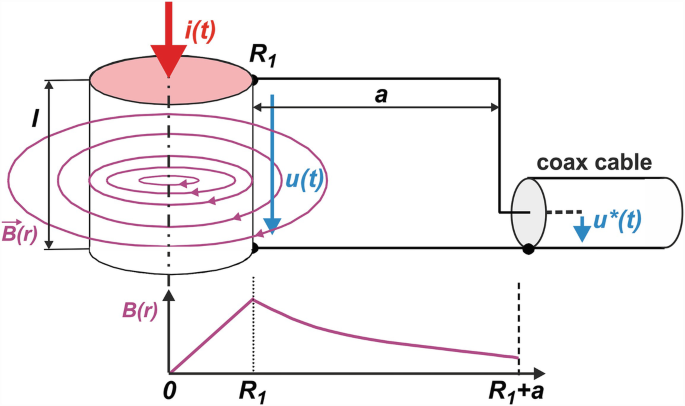
Application Examples

Compressibility factor - Wikipedia

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility Factor Z

Physical Chemistry The Compression Factor (Z) [w/1 example]

Principles of Thermal Physics Lecture notes (KCL) by ucaptd three






:max_bytes(150000):strip_icc()/the-best-gas-grills-under-300-tout-4f01398634694e36a08d44c954c24cd3.jpg)