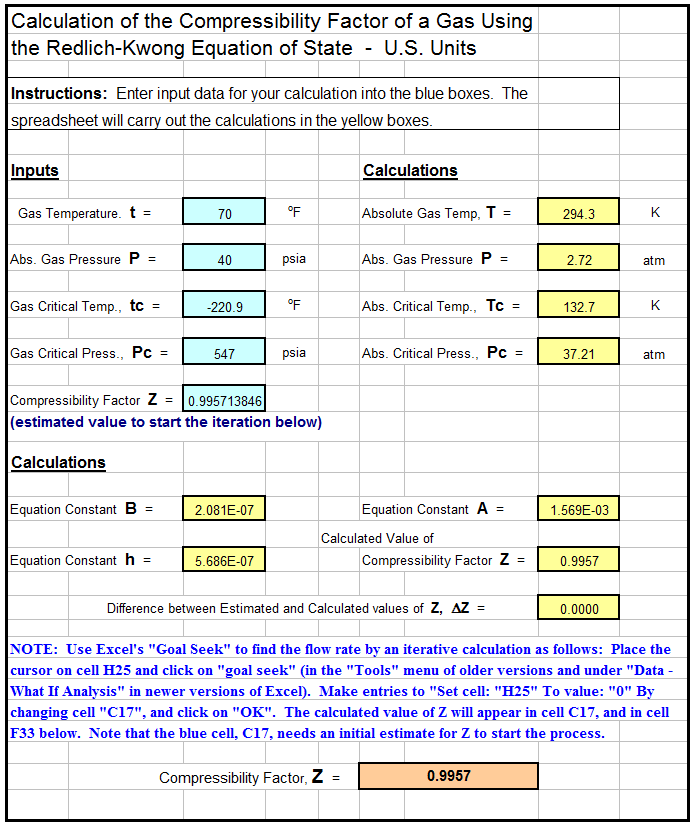
Compressibility factor (Z) for a van der Waals real gas at

Share your videos with friends, family and the world
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
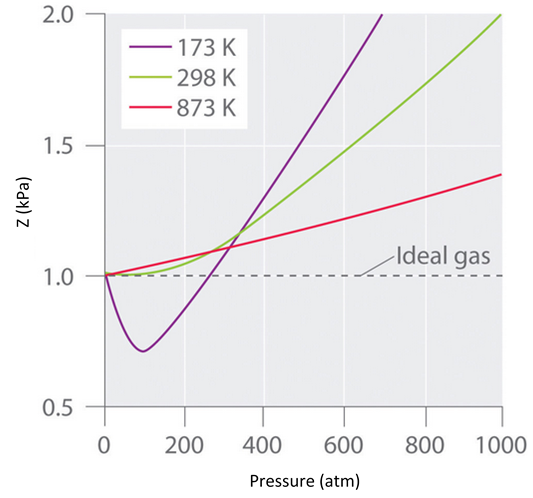
4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts
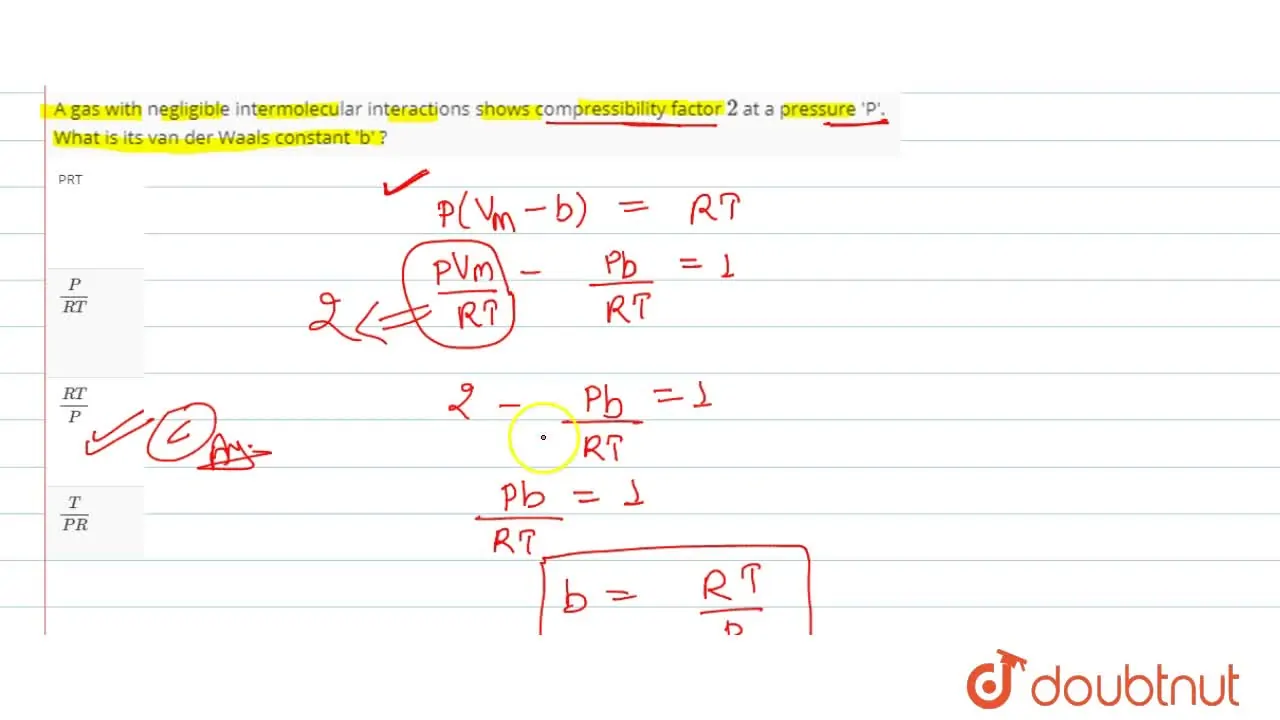
A gas with negligible intermolecular interactions shows compressibilit

A gaseous mixture of 2 moles of A, 3 moles of B, 5 moles of C and 10 m

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
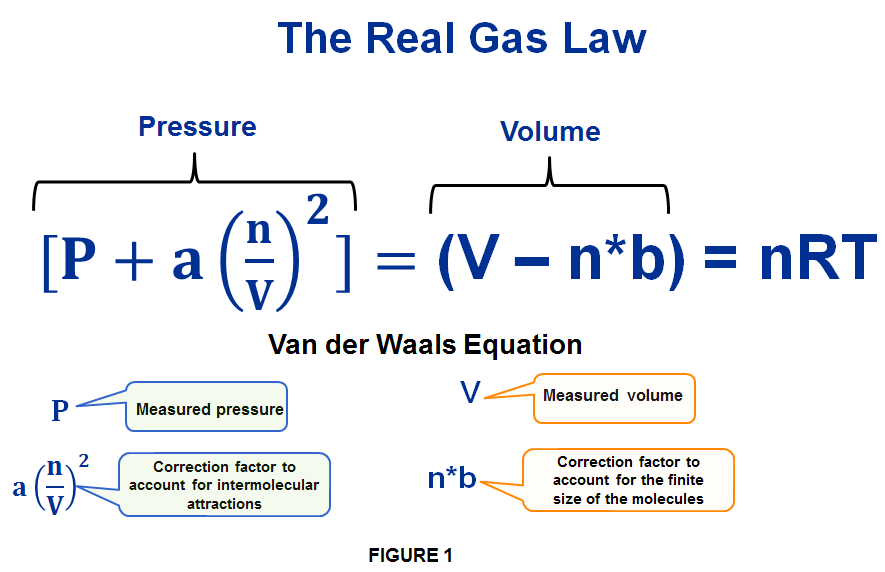
THE 3rd STATE OF MATTER – What is a Real Gas? – Computer Aided Design & The 118 Elements
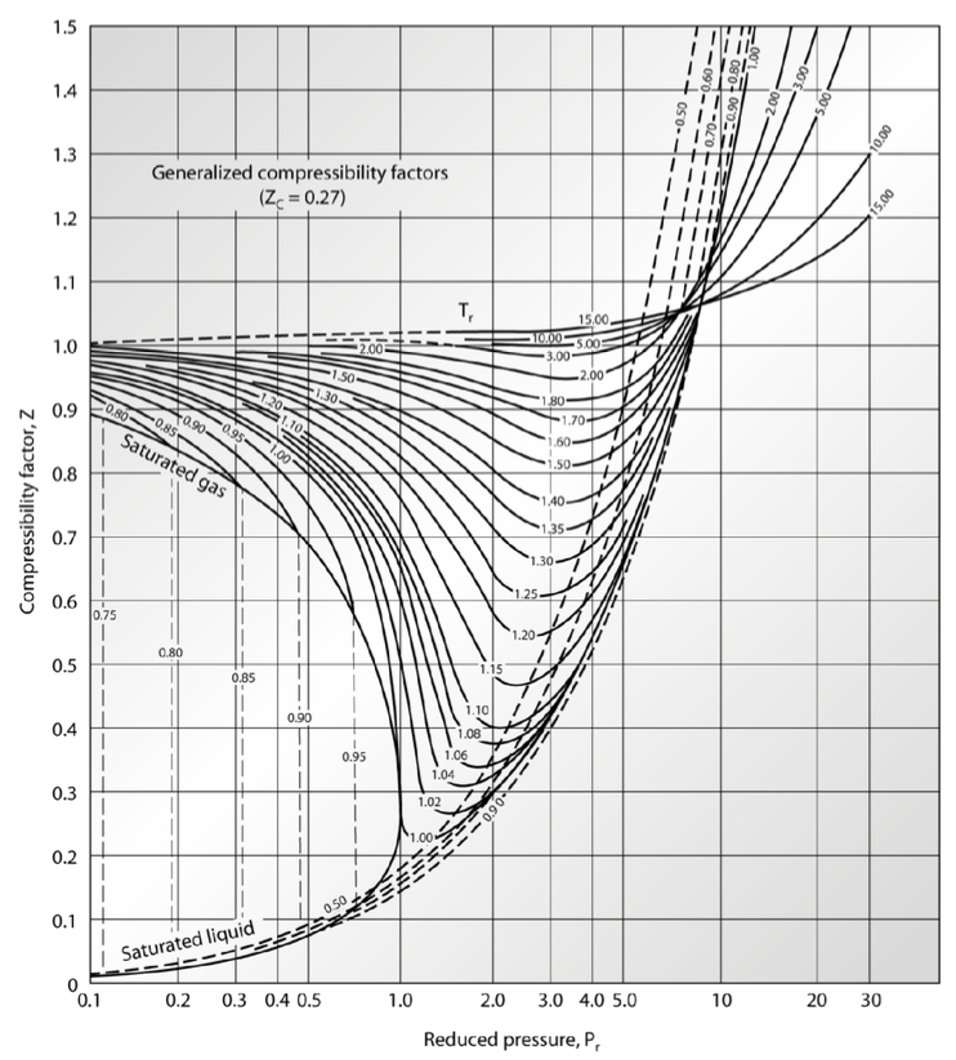
Qin Lab - thermal data
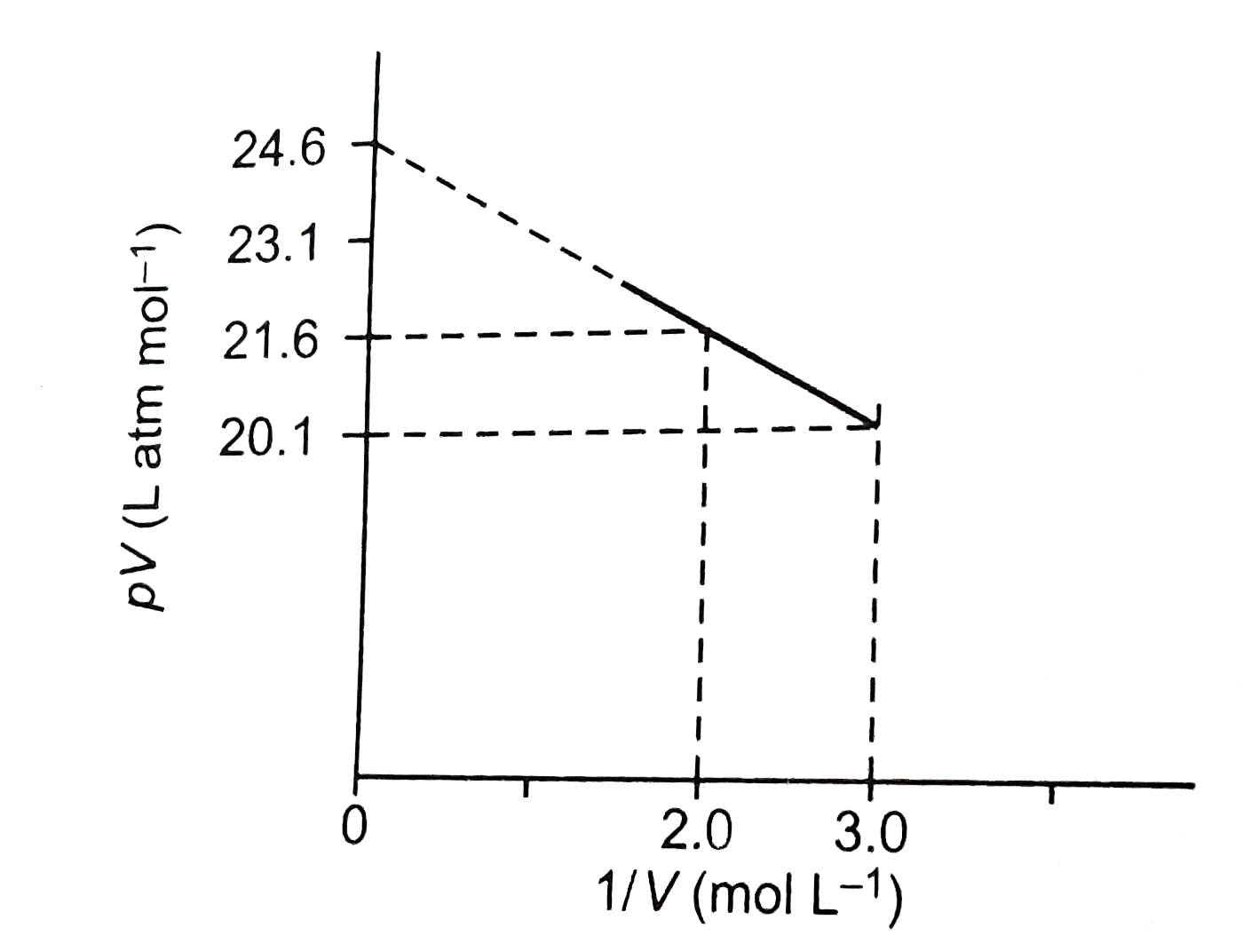
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

Solved The van der Waals equation of state can be used to