The Cottrell Experiment and Diffusion Limitation 3/3

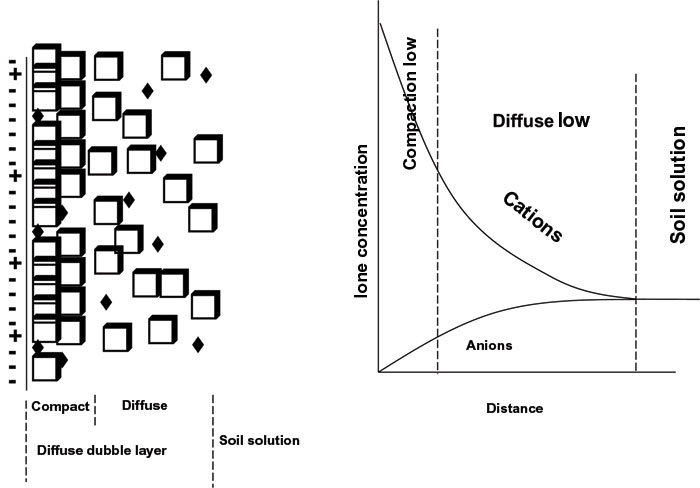
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
Cyclic voltammetry and chronoamperometry: mechanistic tools for organic electrosynthesis - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00706A
support/electrochemical technique

Cobalt Carbonate as an Electrocatalyst for Water Oxidation - Patra - 2020 - Chemistry – A European Journal - Wiley Online Library

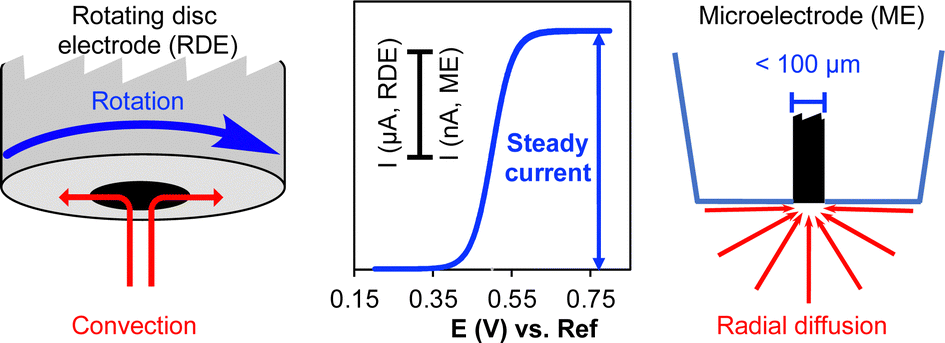
Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields
Crystals, Free Full-Text

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes
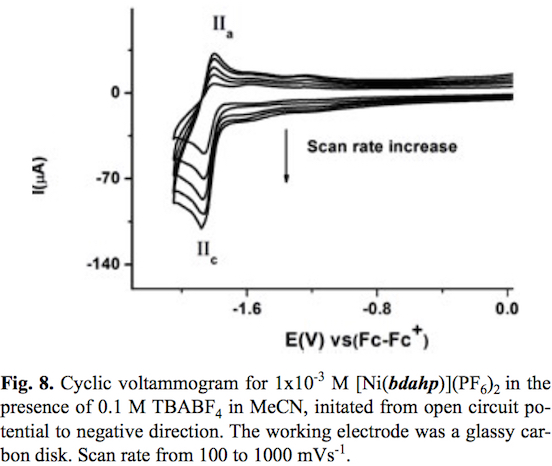
Electrochemical Behavior of Ni(II) Complexes with N2S2 and N6 Ligands as Potential Catalysts in Hydrogen Evolution Reaction

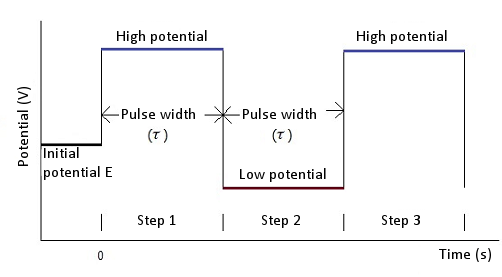
Basic potential step and sweep methods

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes

The interpretation of small molecule diffusion coefficients: Quantitative use of diffusion-ordered NMR spectroscopy - ScienceDirect
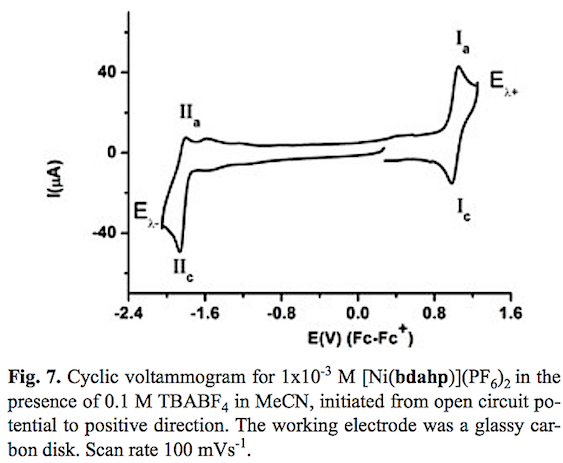
Electrochemical Behavior of Ni(II) Complexes with N2S2 and N6 Ligands as Potential Catalysts in Hydrogen Evolution Reaction

Phase Transformation Lecture 3
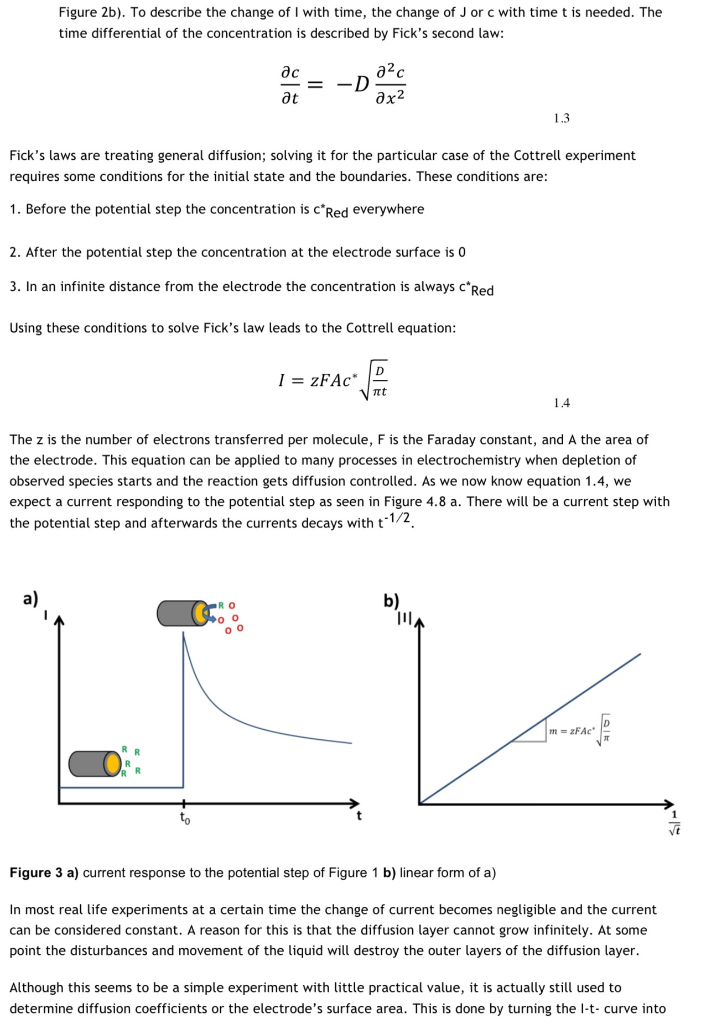
Figure 1.1: Cottrell experiment in KCl solution with