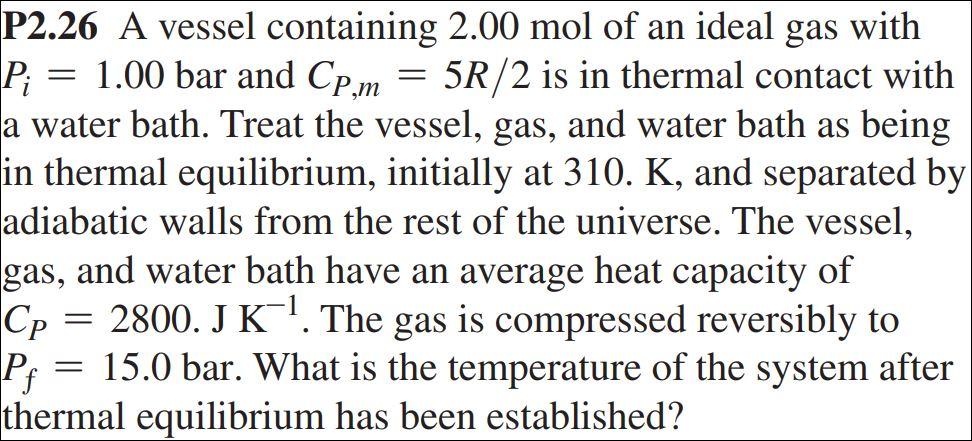
Solved P2.26 A vessel containing 2.00 mol of an ideal gas
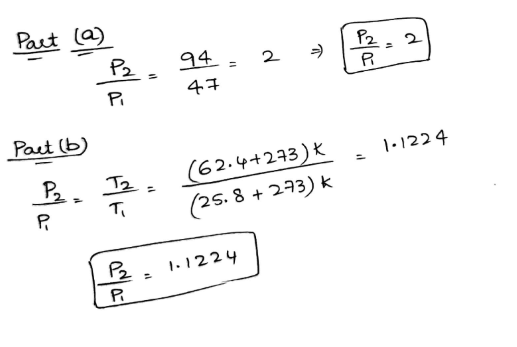
Answered: The volume of an ideal gas is held…
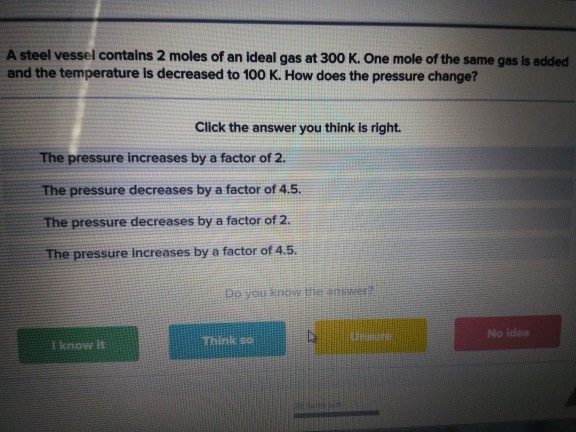
Solved A steel vessel contains 2 moles of an ideal gas at

latent heat or vaporizatul UI Wave - Huurvoo Figure (26-E11) shows a cylindrical tube of volume V with adiabatic walls containing an ideal gas. The internal energy of this ideal gas is

A gaseous mixture enclosed in a vessel of volume V consists of one mole of a gas A with γ=5 / 3 a
Solved] can you please explain with steps. v (m3)= 0.04 V' (m3)=0.017 P
What thermodynamic processes can use the ideal gas approximation
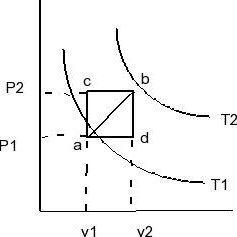
An ideal gas for which cv = 5R/2 is taken from point
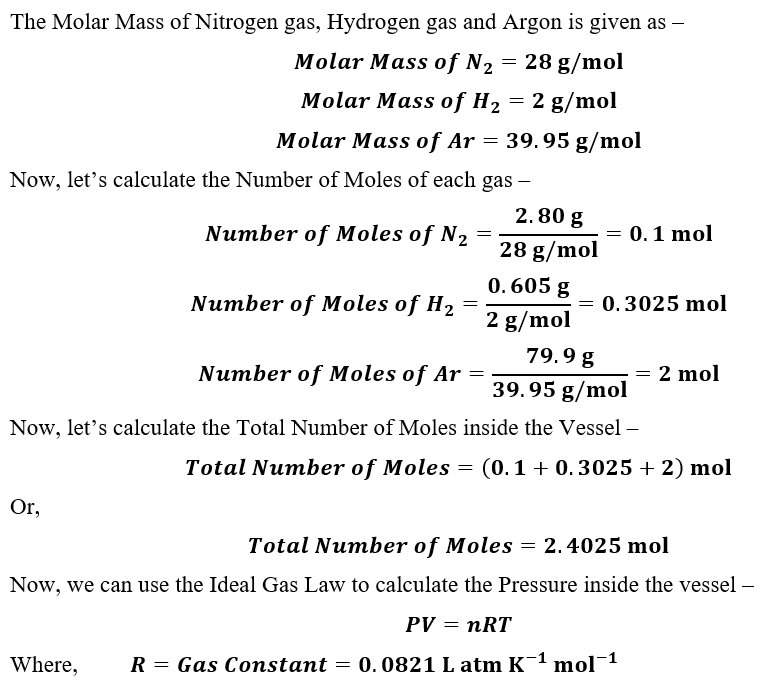
Answered: A vessel with a volume of 26.9 L…

Answered: 25.0 L of an ideal gas at 278 K and…

Given below are two statements, Statement-I: When μ amount of an ideal gas undergoes adiabatic

SOLVED: A sample consisting of 2.00 mol of perfect gas molecules, for which CV,m = 5/2R, initially at p1 = 111 kPa and T1 = 277 K, is heated reversibly to 356

Thermodynamics: An Engineering Approach - 5th Edition - Part I by

An ideal gas, 𝐶𝑝=7/2 𝑅, is heated in a steady-flow heat exchanger from 70℃ to 190℃. Rasayanist







