Sacituzumab Earns Regular FDA Approval for TNBC - NCI

Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.

Targeting Triple-negative Breast Cancer
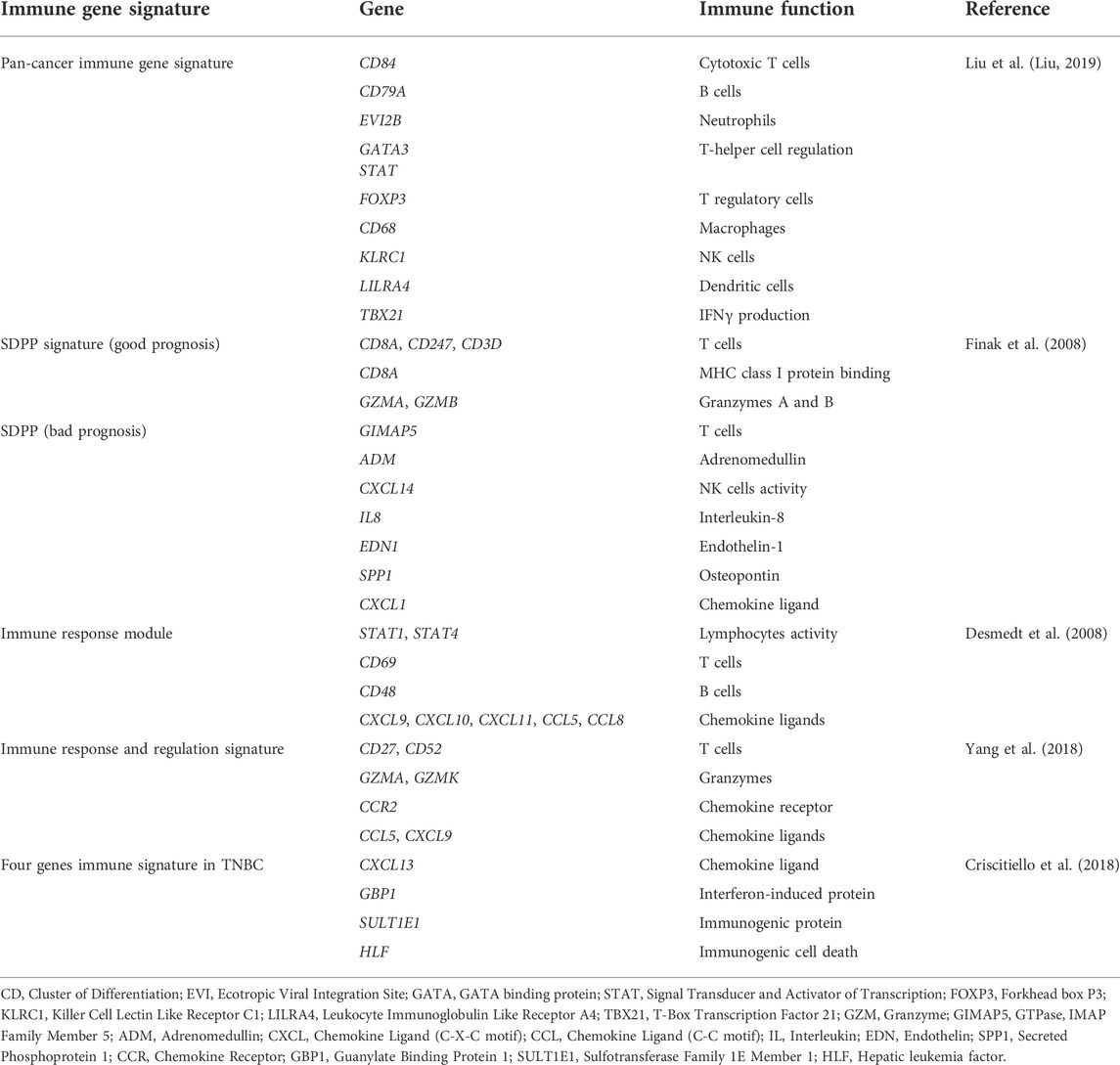
Frontiers Immunotherapy in triple-negative breast cancer: Insights into tumor immune landscape and therapeutic opportunities

Clinical Trials for Metastatic Triple-Negative Breast Cancer

Elina Armani on LinkedIn: #nanoparticles #drugdelivery #sln
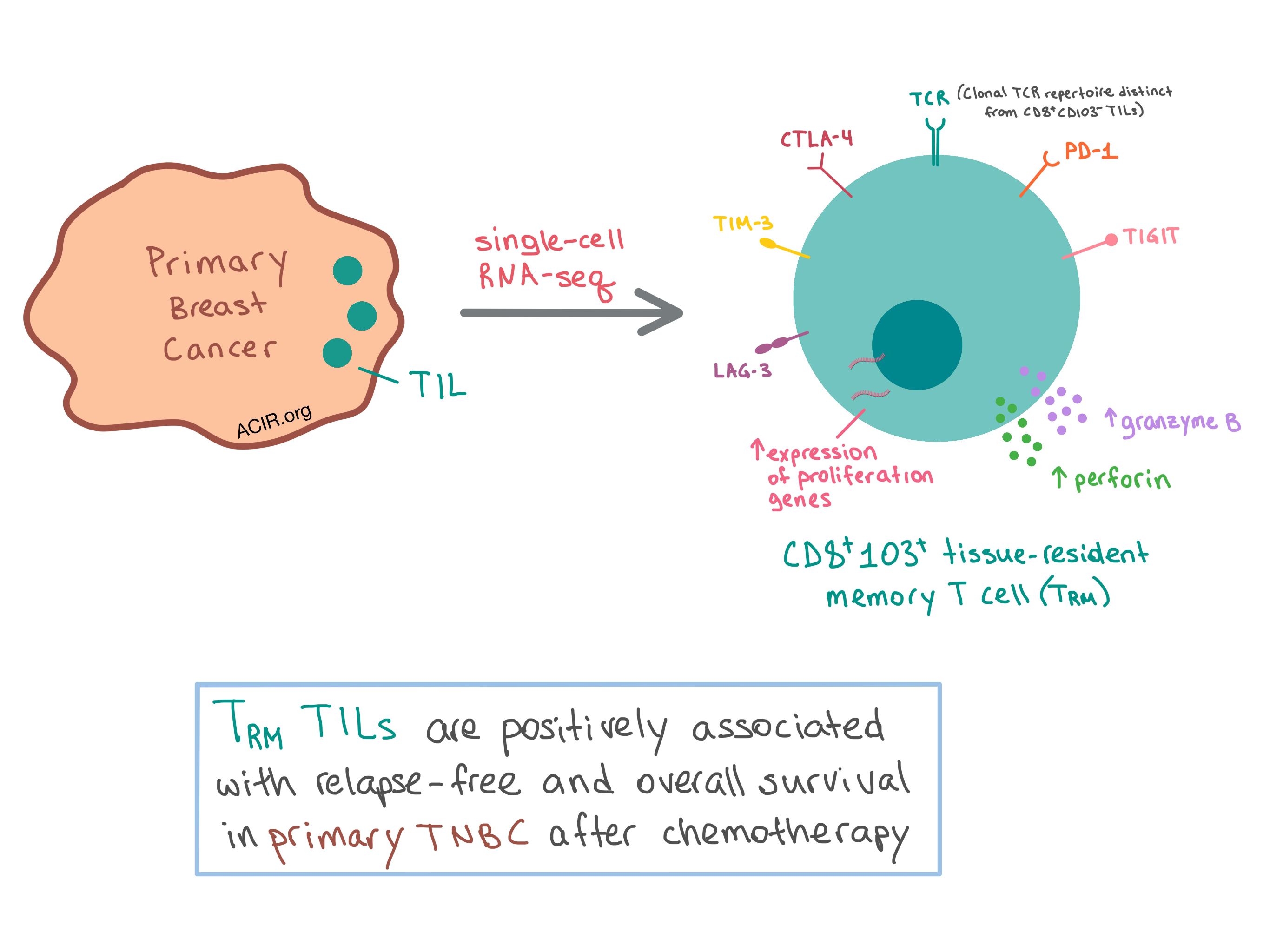
Sacituzumab Earns Regular FDA Approval For TNBC NCI
Determination by IC 50 of sacituzumab govitecan cytotoxicity compared

Sacituzumab Earns Regular FDA Approval for TNBC - NCI

Triple negative breast cancer: Pitfalls and progress

Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer—phase 3 ASCENT study subanalysis

FDA Grants Full Approval to Trodelvy for Triple-Negative Breast Cancer

Sacituzumab govitecan in metastatic triple negative breast cancer







