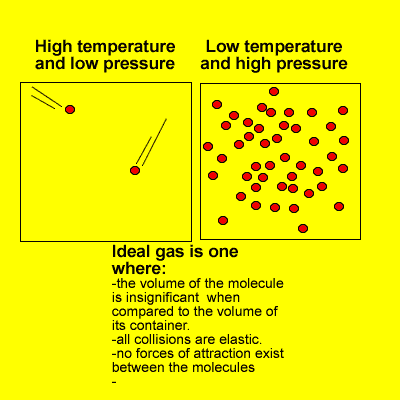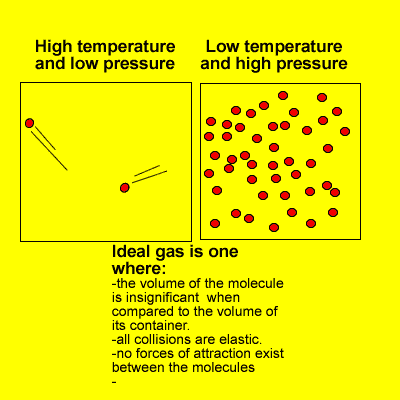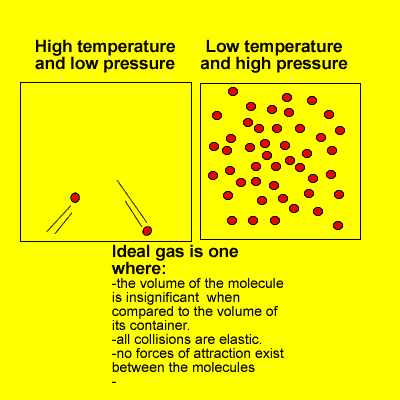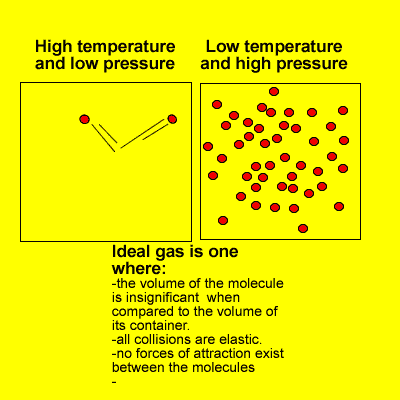
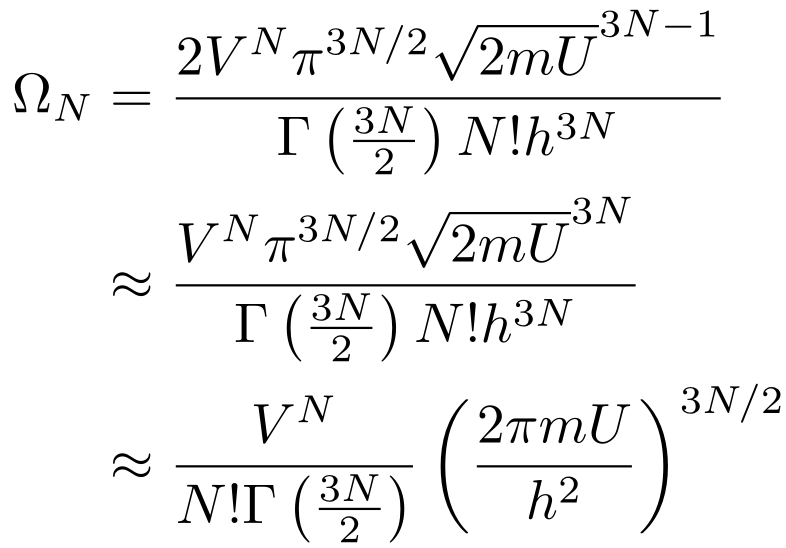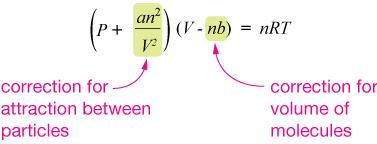Ideal Gas Law: Doubling Temperature and Volume
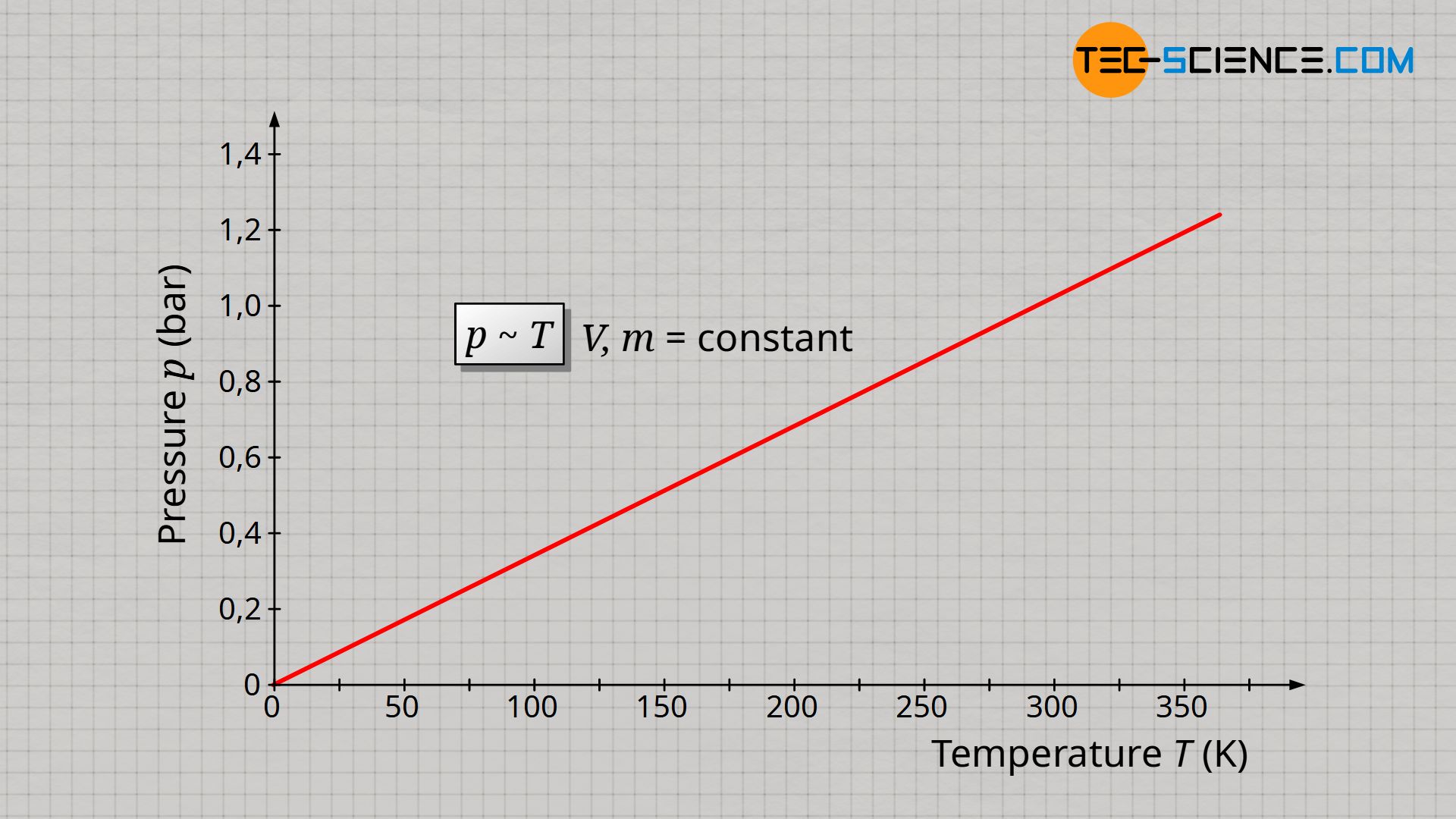
Ideal gas law (explained and derived) - tec-science

THERMODYNAMICS Day 7 of ppt video online download
Ideal Gas Law
If Temperature and Volume are doubled, what happens to the pressure? - Quora

SOLVED: Doubling the initial pressure, at constant temperature under which 1000 mL of a gas was confined causes the volume of the gas to decrease very slightly. According to Boyle's Law, the
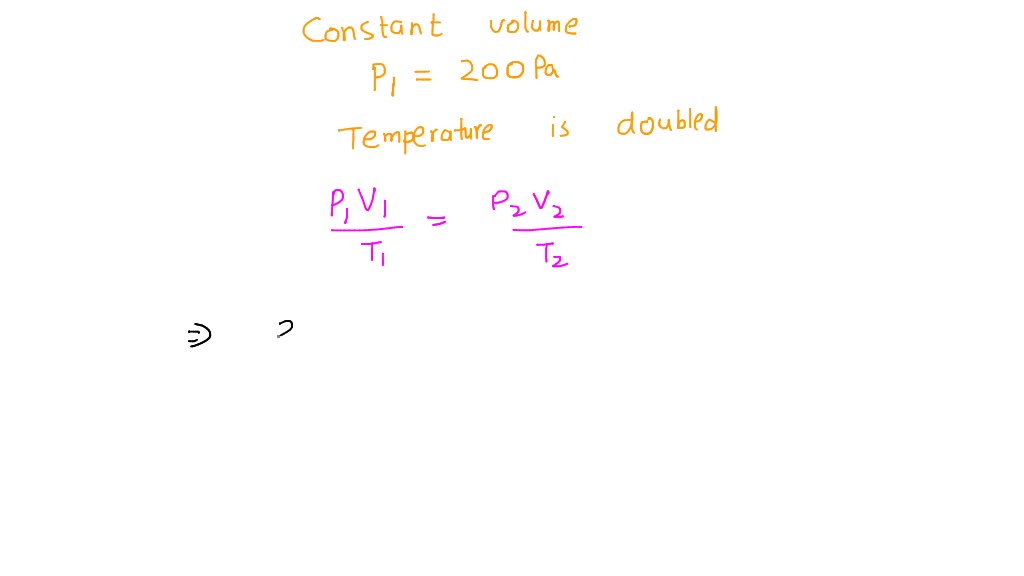
SOLVED: An ideal gas is enclosed in a container of constant volume. The pressure of the gas is initially at 200 Pa. The temperature of the gas is doubled. What is the

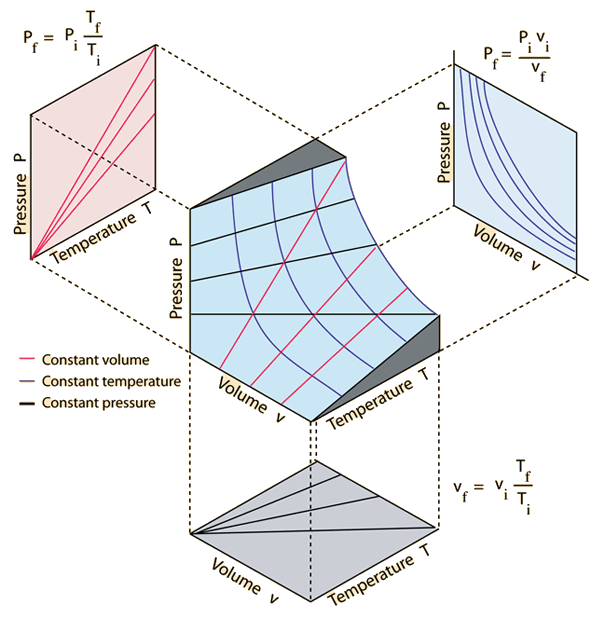
13.3 The Ideal Gas Law

Relationship Between Temperature And Volume
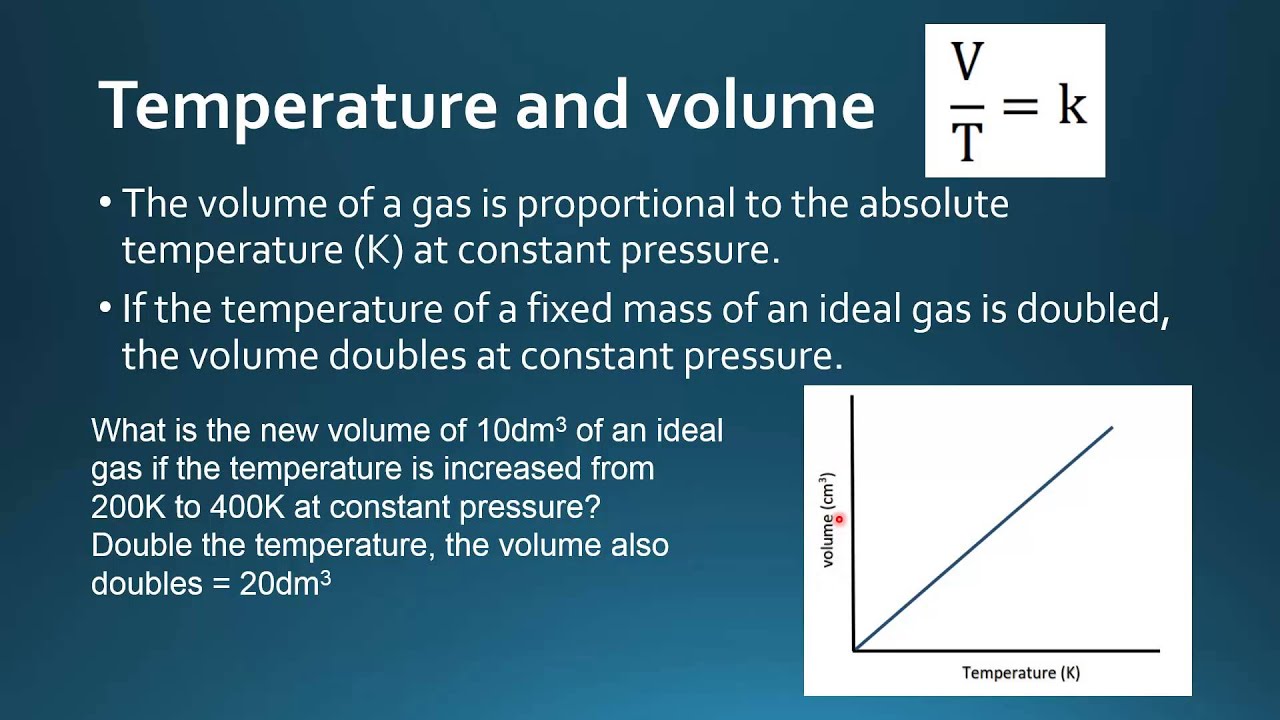
1.4.6 Solve problems involving temperature, pressure and volume for an ideal gas.

Ideal Gas Law - an overview

Gas Laws: Boyle's Law, Charle's Law, Gay-Lussac's Law, Avogadro's Law