2.t 300 K, 36 g of glucose present per litre in itssolution has an
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would be itsconcentration?(1) 11 gl 1(3) 36 gl 1(2) 22 gL 1(4) 42 gL 1
2-t 300 K- 36 g of glucose present per litre in itssolution has an osmotic pressure of 4-98 bar- If theosmotic pressure of solution is 1-52 bar at thesame temperature- what would be itsconcentration-1- 11 gl-1-3- 36 gl-1-2- 22 gL-1-4- 42 gL-1

At 300K 36 g of glucose present per litre in its solution has an osmotic pressure of 4 98 bar - Chemistry - Solutions - 12917865

Solutions: Overview, Questions, Easy Tricks, Rules, Preparation
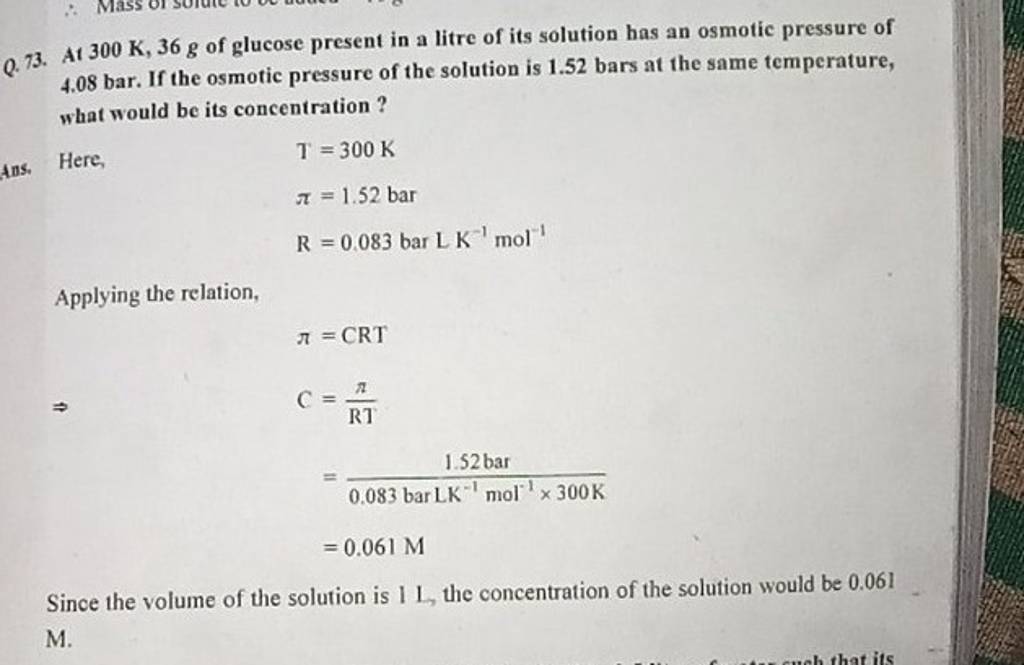
Q.73. At 300 K,36 g of glucose present in a litre of its solution has an ..
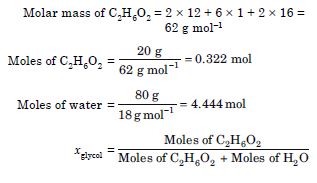
NCERT Solutions for Class 12 Chemistry Solutions

Equal volumes of aqueous 1.00 m glucose (C_{6}H_{12}O_{6}) and 1.00 m sodium chloride solutions are placed on opposite sides of a U-tube, separated by a semipermeable membrane (through which only water can
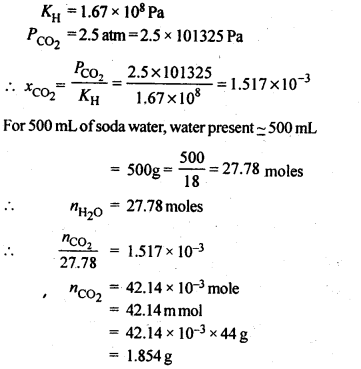
NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would
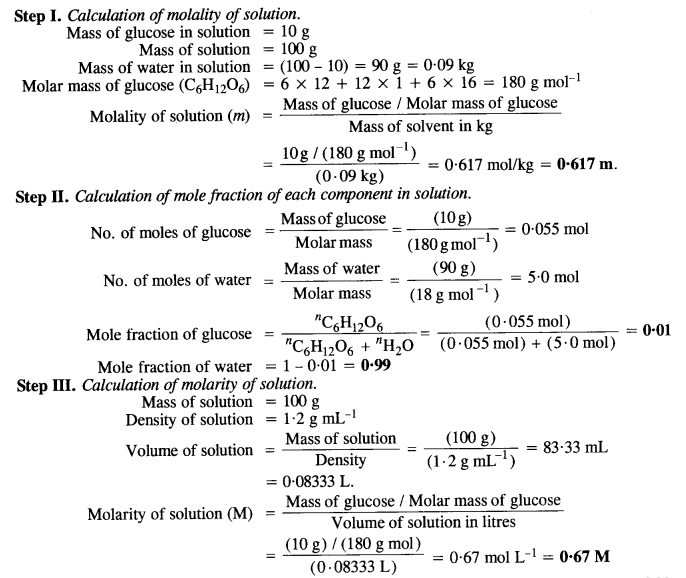
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
At 300 K, 36 g of glucose present in a litre of its solutio - Sarthaks eConnect







