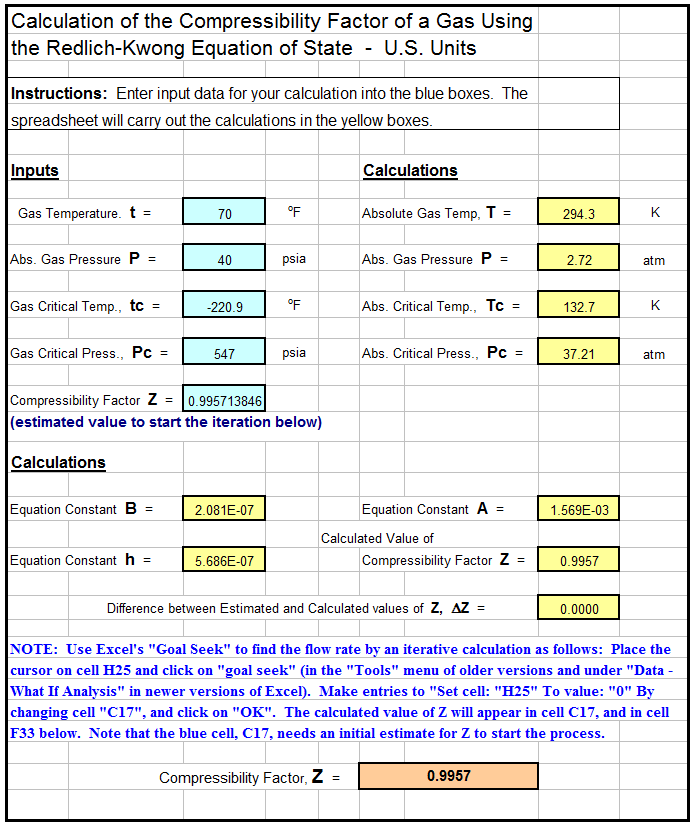
Solved RT B 2. The compressiblity factor for a gas is

Answer to Solved RT B 2. The compressiblity factor for a gas is

Physical Chemistry The Compression Factor (Z) [w/1 example]

Chapter 5 States of Matter - SKY EDUCATIONAL
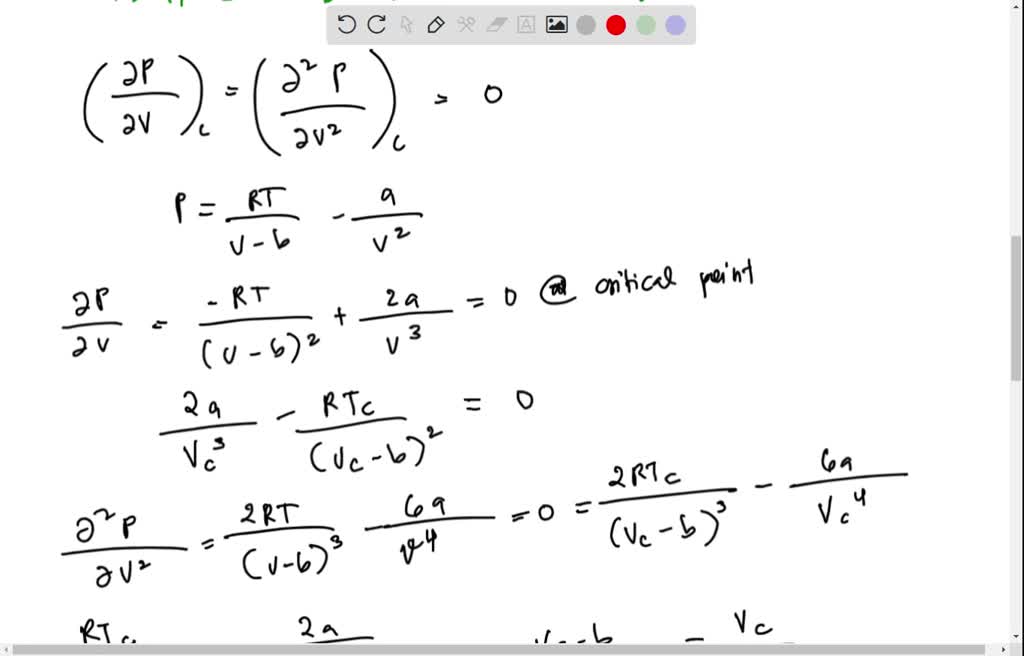
SOLVED: 1) Estimate/ Calculate the critical constants (pc, Vc, and

Compressibility factor - Wikipedia
Viscous effects on real gases in quasi-one-dimensional supersonic convergent divergent nozzle flows, Journal of Fluid Mechanics
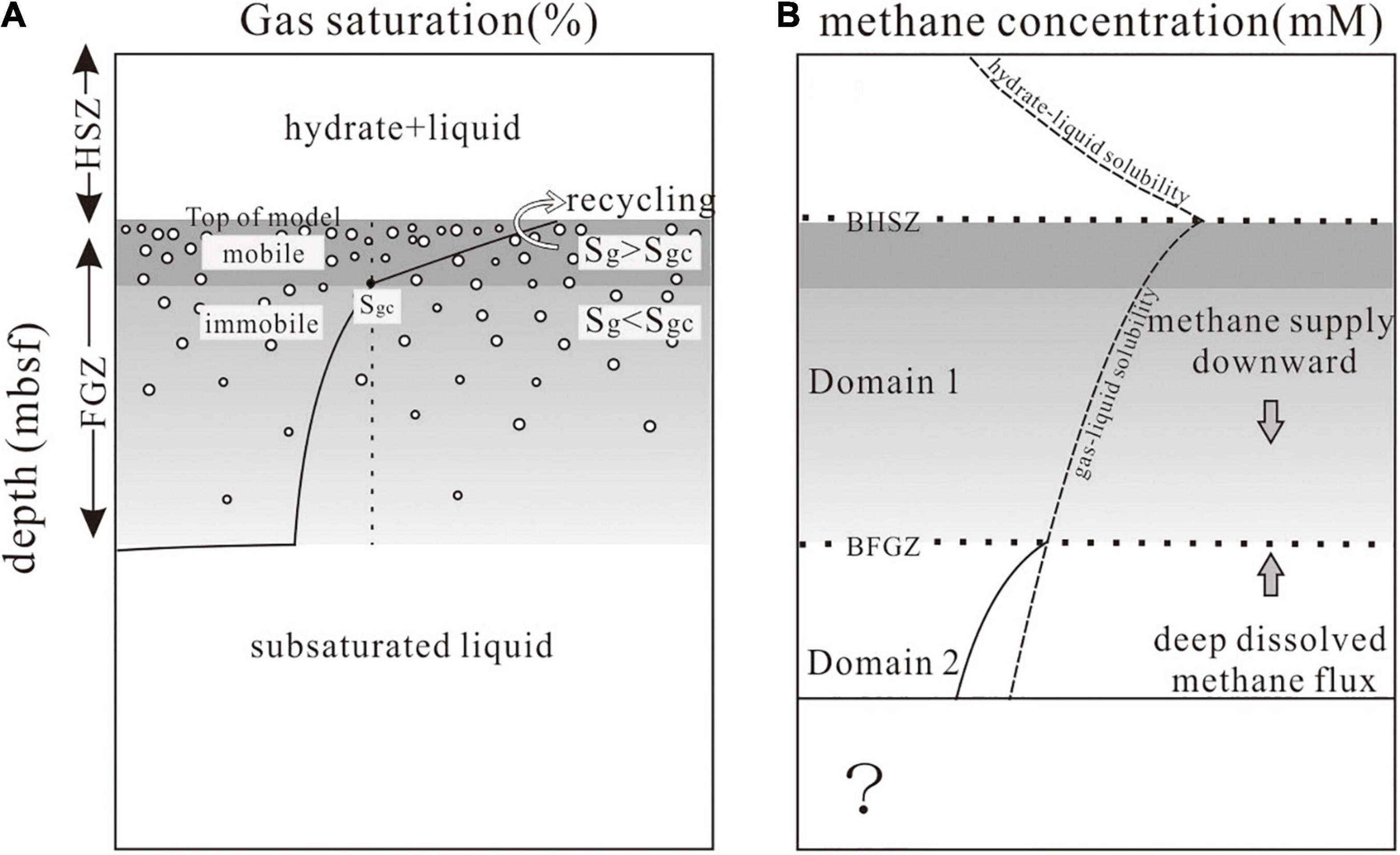
Frontiers A Numerical Model for Determining Deep Methane Flux
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
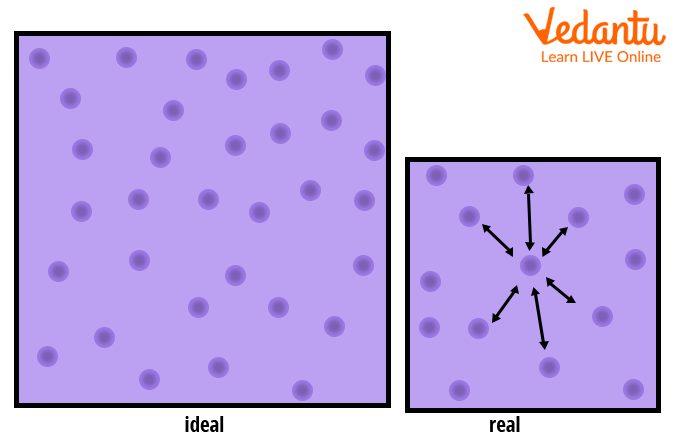
JEE - Compressibility Factor Important Concepts and Tips

The compressibility factor for a real gas is expressed by, z =1+ BP / RT. The value of B at 500 K and 600 bar is 0.0169 L / mol. Find the

Explain how the compression factor varies with pressure and

Compressibility factor for methane.