13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racem..

Solution For 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g)
13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is:
Video solution 1: 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is
Solved 1 35 7 9 11 13 15 2 3 6 7 0 11 14 15 4 5 6 7 12 13 14
Solved J3 Chart Chunks worksheet 1 6 1 9 8 10 11 12 13 14 15
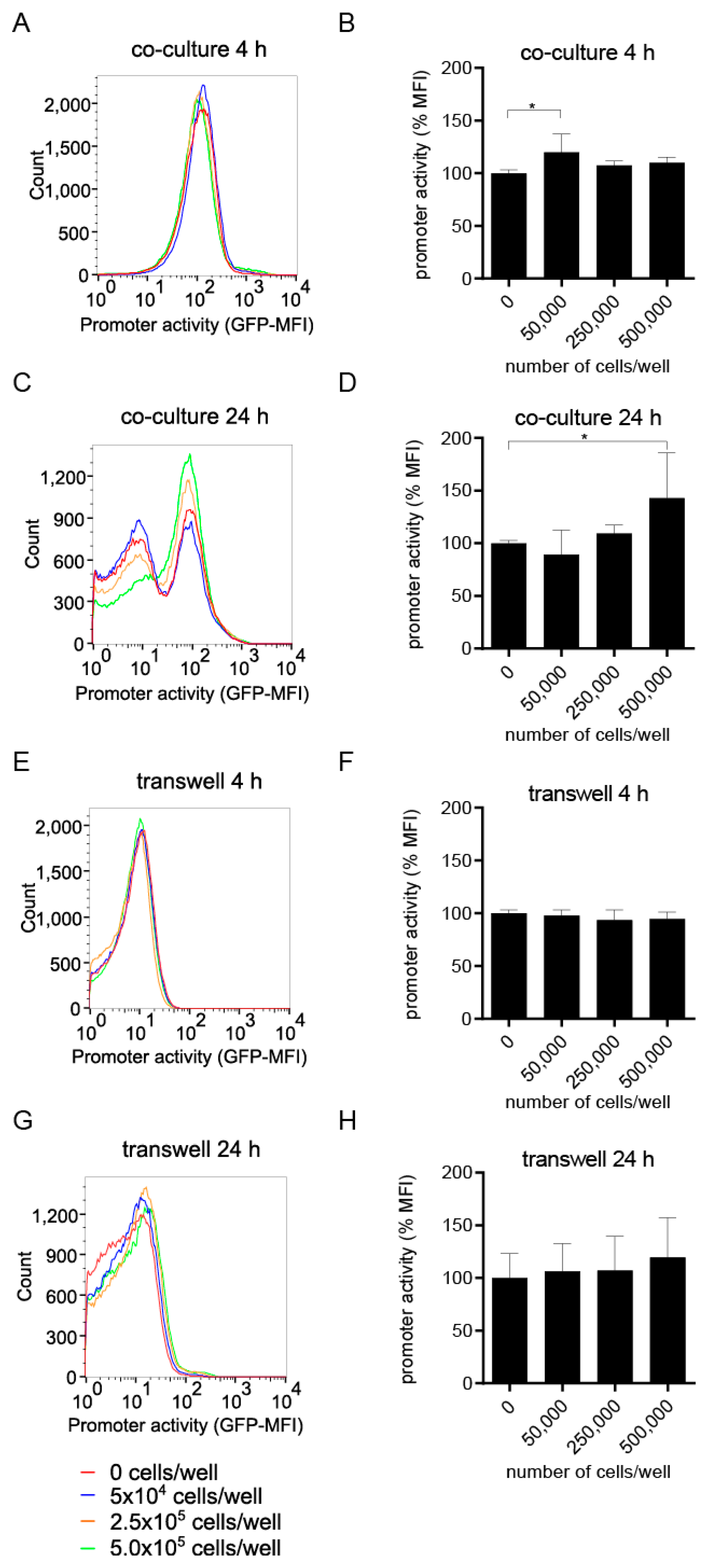
IJMS, Free Full-Text
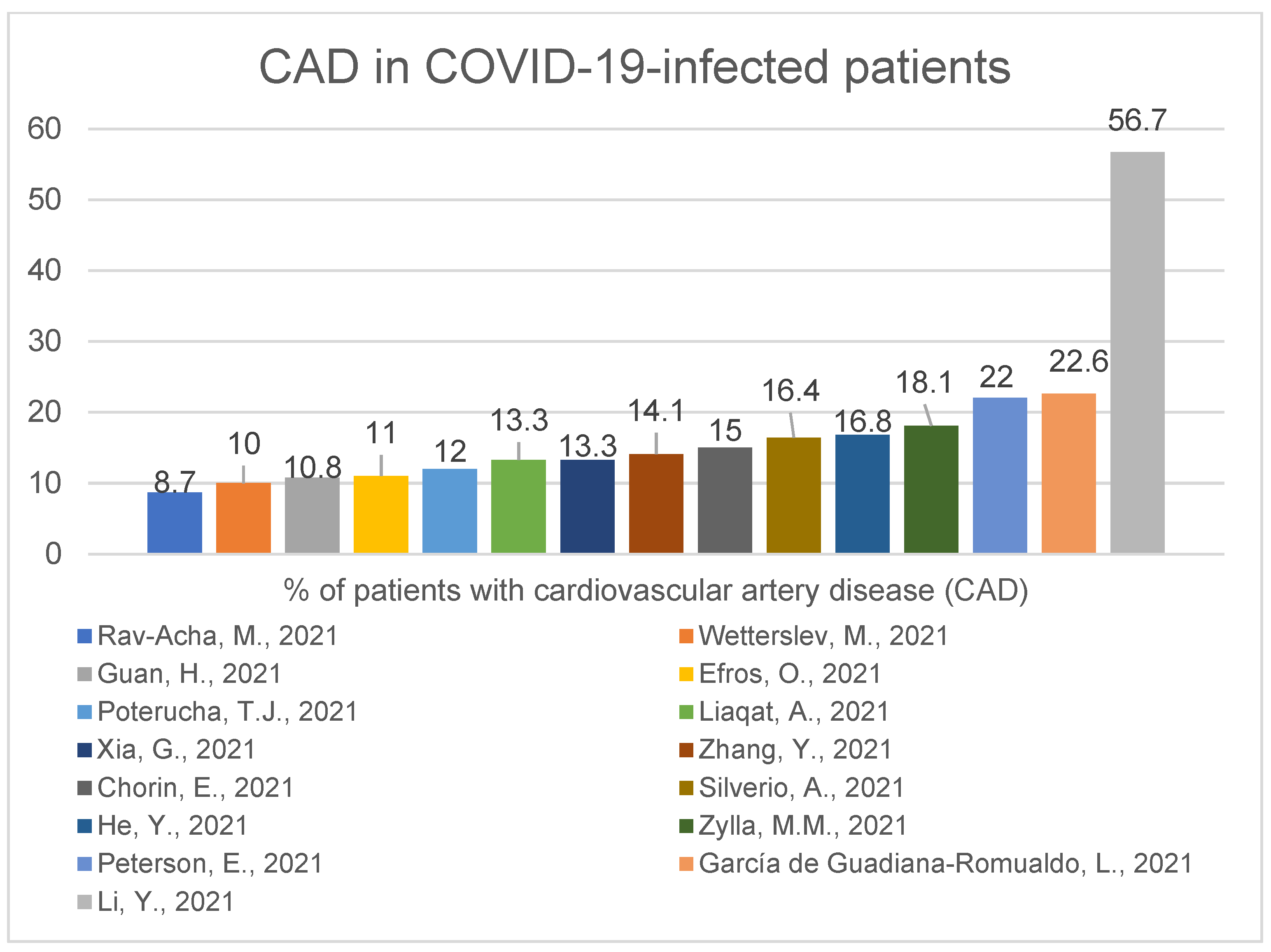
IJERPH, Free Full-Text

Comorbidity, misdiagnoses, and the diagnostic odyssey in patients
PPT - dos PowerPoint Presentation, free download - ID:4486095

Continued 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28

Assobens Magazine #383 by ASSOBENS - Issuu
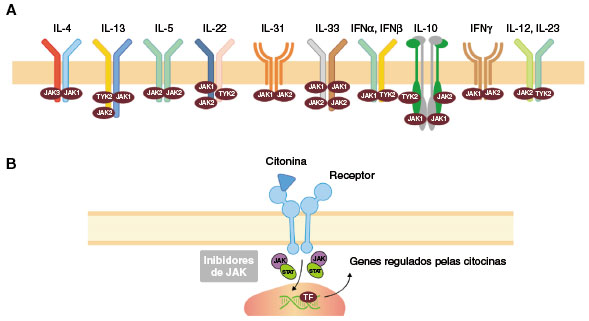
Arquivos de Asma, Alergia e Imunologia - AAAI

exh991-1q21investormeeti

1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
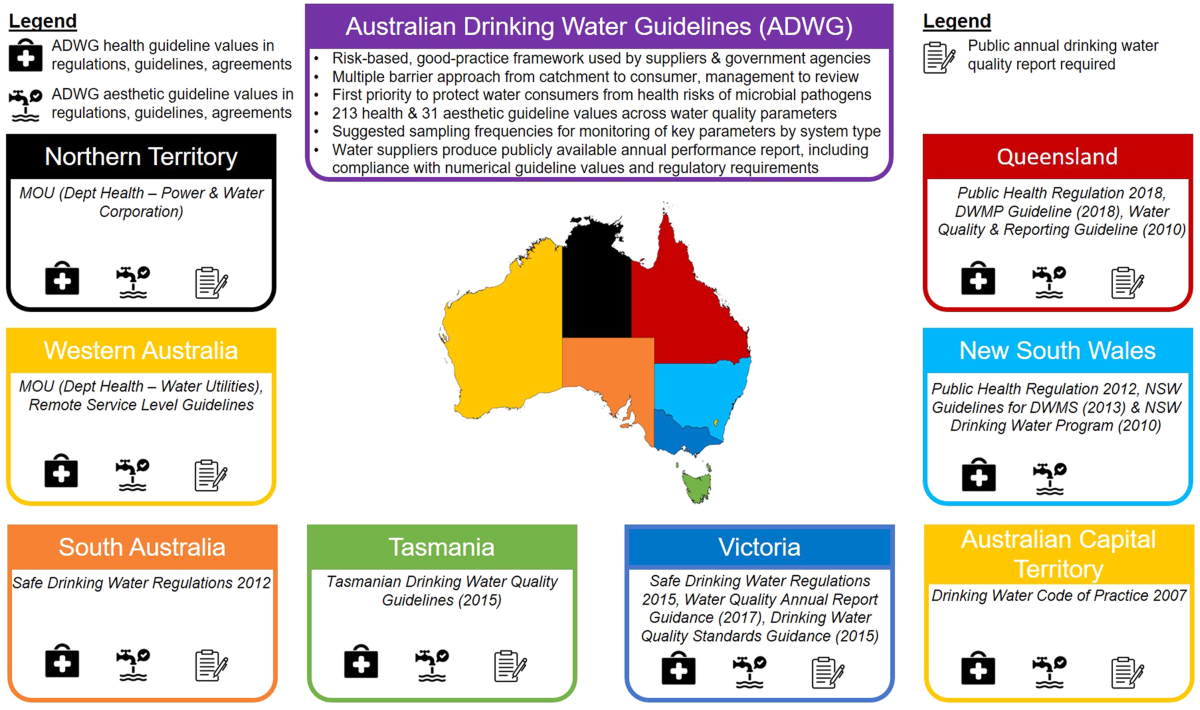
Measuring the gaps in drinking water quality and policy across

Global education digest 2007: comparing education statistics

Abortion Surveillance — United States, 2019

Counting ppt download